The unfulfilled potential of mucosal immunization
- PMID: 35569567
- PMCID: PMC9098804
- DOI: 10.1016/j.jaci.2022.05.002
The unfulfilled potential of mucosal immunization
Abstract
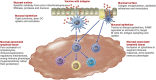
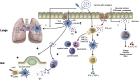
Recent events involving the global coronavirus pandemic have focused attention on vaccination strategies. Although tremendous advances have been made in subcutaneous and intramuscular vaccines during this time, one area that has lagged in implementation is mucosal immunization. Mucosal immunization provides several potential advantages over subcutaneous and intramuscular routes, including protection from localized infection at the site of entry, clearance of organisms on mucosal surfaces, induction of long-term immunity through establishment of central and tissue-resident memory cells, and the ability to shape regulatory responses. Despite these advantages, significant barriers remain to achieving effective mucosal immunization. The epithelium itself provides many obstacles to immunization, and the activation of immune recognition and effector pathways that leads to mucosal immunity has been difficult to achieve. This review will highlight the potential advantages of mucosal immunity, define the barriers to mucosal immunization, examine the immune mechanisms that need to be activated on mucosal surfaces, and finally address recent developments in methods for mucosal vaccination that have shown promise in generating immunity on mucosal surfaces in human trials.
Keywords: Mucosal vaccines; barriers to mucosal immunization; innate lymphoid cells; respiratory infections; sterile immunity; tissue resident memory cells.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

