Apixaban Use in Obese Patients: A Review of the Pharmacokinetic, Interventional, and Observational Study Data
- PMID: 35570249
- PMCID: PMC9618533
- DOI: 10.1007/s40256-022-00524-x
Apixaban Use in Obese Patients: A Review of the Pharmacokinetic, Interventional, and Observational Study Data
Abstract
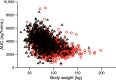
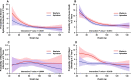
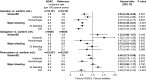
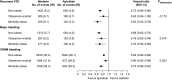
Relatively little is known about the influence of extreme body weight on the pharmacokinetics (PK), pharmacodynamics (PD), efficacy, and safety of drugs used in many disease states. While direct oral anticoagulants (DOACs) have an advantage over warfarin in that they do not require routine drug monitoring, some may regard this convenience as less compelling in obese patients. Some consensus guidelines discourage using DOACs in patients weighing > 120 kg or with a body mass index > 35-40 kg/m2, given a sparsity of available data in this population and the concern that fixed dosing in obese patients might lead to decreased drug exposure and lower efficacy. Per the prescribing information, apixaban does not require dose adjustment in patients weighing above a certain threshold (e.g., ≥ 120 kg). Data from healthy volunteers and patients with nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) have shown that increased body weight has a modest effect on apixaban's PK. However, the paucity of exposure data in individuals > 120 kg and the lack of guideline consensus on DOAC use in obese patients continue to raise concerns about potential decreased drug exposure at extreme weight. This article is the first to comprehensively review the available PK data in obese individuals without NVAF or VTE, and PK, PD, efficacy, effectiveness, and safety data for apixaban in obese patients with either NVAF or VTE, including subgroup analyses across randomized controlled trials and observational (real-world) studies. These data suggest that obesity does not substantially influence the efficacy, effectiveness, or safety of apixaban in these patients. Trial Registration ARISTOTLE: NCT00412984; AVERROES: NCT00496769; AMPLIFY: NCT00643201; AMPLIFY-EXT: NCT00633893; ADVANCE-1: NCT00371683; ADVANCE-2: NCT00452530; ADVANCE-3: NCT00423319 Apixaban Use in Obese Patients: A Review of the Pharmacokinetic, Interventional, and Observational Study Data (MP4 161.22 MB).
© 2022. © Pfizer.
Conflict of interest statement
MJJ, WB, RWD, AJQ, and CR are employees and shareholders of Pfizer. MC is an employee of Pfizer. PG, JO, and SJM are employees and shareholders of Bristol Myers Squibb.
Figures



References
-
- World Health Organization. WHO obesity and overweight fact sheet. 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 31 Aug 2020.
-
- Centers for Disease Control and Prevention. Defining adult overweight & obesity. 2021. https://www.cdc.gov/obesity/adult/defining.html. Accessed 29 Mar 2021.
-
- University of Rochester. What is morbid obesity? 2020. https://www.urmc.rochester.edu/highland/bariatric-surgery-center/journey.... Accessed 24 June 2021.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

