Cytotoxicity of 3D-printed, milled, and conventional oral splint resins to L929 cells and human gingival fibroblasts
- PMID: 35570327
- PMCID: PMC9209804
- DOI: 10.1002/cre2.592
Cytotoxicity of 3D-printed, milled, and conventional oral splint resins to L929 cells and human gingival fibroblasts
Abstract
Objectives: Evidence on the biocompatibility of three-dimensional (3D)-printed and milled resins for oral splints is limited. This in vitro study assessed the influence of the manufacturing method on the cytotoxicity of oral splint resins on L929 cells and human gingival fibroblasts (GF1).
Materials and methods: Standardized specimens of four 3D-printed, two-milled, one-thermoformed, and one-pressed splint resin were incubated with L929 and GF1 cells for 24 h. Immunofluorescence and WST-8 assay were performed to evaluate cytotoxic effects. One-way analysis of variance and Tukey's multiple comparison test were applied with the variables "splint resin" and "manufacturing method" (p < .05).
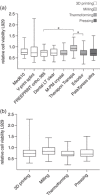
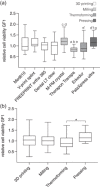
Results: Immunofluorescence showed attachment of L929 and GF1 cells to the splint resins. Irrespective of the manufacturing method, the WST-8 assay revealed significant differences between splint resins for the viability of L929 and GF1 cells. L929 cells generally showed lower viability rates than GF1 cells. The evaluation of cell viability by the manufacturing method showed no significant differences between 3D printing, milling, and conventional methods.
Conclusions: The cytotoxic effects of 3D-printed, milled, and conventional oral splint resins were similar, indicating minor influence of the manufacturing method on biocompatibility. Cytotoxicity of the resins was below a critical threshold in GF1 cells. The chemical composition might be more crucial than the manufacturing method for the biocompatibility of splint resins.
Keywords: 3D printing; cytotoxicity; milling; splint resins.
© 2022 The Authors. Clinical and Experimental Dental Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
In vitro biocompatibility testing of 3D printing and conventional resins for occlusal devices.J Dent. 2022 Aug;123:104163. doi: 10.1016/j.jdent.2022.104163. Epub 2022 May 14. J Dent. 2022. PMID: 35577252
-
Elution behavior of a 3D-printed, milled and conventional resin-based occlusal splint material.Dent Mater. 2021 Apr;37(4):701-710. doi: 10.1016/j.dental.2021.01.024. Epub 2021 Feb 27. Dent Mater. 2021. PMID: 33648744
-
Influence of the Manufacturing Method on the Adhesion of Candida albicans and Streptococcus mutans to Oral Splint Resins.Polymers (Basel). 2021 May 11;13(10):1534. doi: 10.3390/polym13101534. Polymers (Basel). 2021. PMID: 34064561 Free PMC article.
-
Biocompatibility of 3D-Printed Dental Resins: A Systematic Review.Cureus. 2024 Jan 5;16(1):e51721. doi: 10.7759/cureus.51721. eCollection 2024 Jan. Cureus. 2024. PMID: 38318586 Free PMC article. Review.
-
Physical and Mechanical Properties of 3D-Printed Provisional Crowns and Fixed Dental Prosthesis Resins Compared to CAD/CAM Milled and Conventional Provisional Resins: A Systematic Review and Meta-Analysis.Polymers (Basel). 2022 Jun 30;14(13):2691. doi: 10.3390/polym14132691. Polymers (Basel). 2022. PMID: 35808735 Free PMC article. Review.
Cited by
-
Disparity in the Influence of Implant Provisional Materials on Human Gingival Fibroblasts with Different Phases of Cell Settlement: An In Vitro Study.Int J Mol Sci. 2023 Dec 21;25(1):123. doi: 10.3390/ijms25010123. Int J Mol Sci. 2023. PMID: 38203293 Free PMC article.
-
Therapy for Temporomandibular Disorders: 3D-Printed Splints from Planning to Evaluation.Dent J (Basel). 2023 May 8;11(5):126. doi: 10.3390/dj11050126. Dent J (Basel). 2023. PMID: 37232777 Free PMC article.
-
Biocompatibility of 3D-printed vs. thermoformed and heat-cured intraoral appliances.Front Bioeng Biotechnol. 2024 Oct 29;12:1453888. doi: 10.3389/fbioe.2024.1453888. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39534672 Free PMC article.
-
Design of Liposome Formulations for CRISPR/Cas9 Enzyme Immobilization: Evaluation of 5-Alpha-Reductase Enzyme Knockout for Androgenic Disorders.ACS Omega. 2023 Nov 20;8(48):46101-46112. doi: 10.1021/acsomega.3c07138. eCollection 2023 Dec 5. ACS Omega. 2023. PMID: 38075788 Free PMC article.
-
Analysis of Gingival Fibroblasts Behaviour in the Presence of 3D-Printed versus Milled Methacrylate-Based Dental Resins-Do We Have a Winner?J Funct Biomater. 2024 May 28;15(6):147. doi: 10.3390/jfb15060147. J Funct Biomater. 2024. PMID: 38921521 Free PMC article.
References
-
- Alghazzawi, T. F. (2016, April 1). Advancements in CAD/CAM technology: Options for practical implementation. Journal of Prosthodontic Research, 60(2), 72–84. - PubMed
-
- Ali, A. , Bates, J. F. , Reynolds, A. J. , & Walker, D. M. (1986, December 20). The burning mouth sensation related to the wearing of acrylic dentures: An investigation. British Dental Journal, 161(12), 444–447. - PubMed
-
- Alifui‐Segbaya, F. , Bowman, J. , White, A. R. , George, R. , & Fidan, I. (2019, December). Characterization of the double bond conversion of acrylic resins for 3D printing of dental prostheses. Compendium of Continuing Education in Dentistry, 40(10), e7–e11. - PubMed
-
- Ata, S. O. , & Yavuzyilmaz, H. (2009, November). In vitro comparison of the cytotoxicity of acetal resin, heat‐polymerized resin, and auto‐polymerized resin as denture base materials. Journal of Biomedical Materials Research, Part B: Applied Biomaterials, 91(2), 905–909. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

