Statistic Copolymers Working as Growth Factor-Binding Mimics of Fibronectin
- PMID: 35570405
- PMCID: PMC9313494
- DOI: 10.1002/advs.202200775
Statistic Copolymers Working as Growth Factor-Binding Mimics of Fibronectin
Abstract
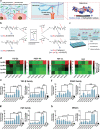
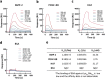
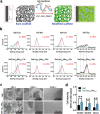
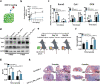
Growth factors (GFs) play important roles in biological system and are widely used in tissue regeneration. However, their application is greatly hindered by short in vivo lifetime of GFs. GFs are bound to fibronectin dynamically in the extracellular matrix, which inspired the authors to mimic the GF binding domain of fibronectin and design GF-binding amphiphilic copolymers bearing positive charges. The optimal amino acid polymer can bind to a variety of representative GFs, such as bone morphogenetic protein-2 (BMP-2) and TGF-β1 from the transforming growth factor-β superfamily, PDGF-AA and PDGF-BB from the platelet-derived growth factor family, FGF-10 and FGF-21 from the fibroblast growth factor family, epidermal growth factor from the EGF family and hepatocyte growth factor from the plasminogen-related growth factor family, with binding affinities up to the nanomolar level. 3D scaffolds immobilized with the optimal copolymer enable sustained release of loaded BMP-2 without burst release and significantly enhances the in vivo function of BMP-2 for bone formation. This strategy opens new avenues in designing GF-binding copolymers as synthetic mimics of fibronectin for diverse applications.
Keywords: fibronectin mimicking; growth factor binding; statistic copolymers.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
R.L. and W.Z. are co‐inventors on a patent application covering reported materials and application to bind growth factors. All remaining authors declare no competing interests.
Figures

References
-
- Rapraeger A. C., Krufka A., Olwin B. B., Science 1991, 252, 1705. - PubMed
-
- a) Ehrbar M., Djonov V. G., Schnell C., Tschanz S. A., Martiny‐Baron G., Schenk U., Wood J., Burri P. H., Hubbell J. A., Zisch A. H., Circ. Res. 2004, 94, 1124; - PubMed
- b) Puccinelli T. J., Bertics P. J., Masters K. S., Acta Biomater. 2010, 6, 3415; - PMC - PubMed
- c) Hudalla G. A., Koepsel J. T., Murphy W. L., Adv. Mater. 2011, 23, 5415; - PMC - PubMed
- d) Chu H., Gao J., Chen C. W., Huard J., Wang Y., Proc. Natl. Acad. Sci. USA 2011, 108, 13444; - PMC - PubMed
- e) Samorezov J. E., Alsberg E., Adv. Drug Del. Rev. 2015, 84, 45; - PMC - PubMed
- f) Schwab E. H., Pohl T. L. M., Haraszti T., Schwaerzer G. K., Hiepen C., Spatz J. P., Knaus P., Cavalcanti‐Adam E. A., Nano Lett. 2015, 15, 1526; - PubMed
- g) Seo B.‐B., Koh J.‐T., Song S.‐C., Biomaterials 2017, 122, 91; - PubMed
- h) Tabisz B., Schmitz W., Schmitz M., Luehmann T., Heusler E., Rybak J.‐C., Meinel L., Fiebig J. E., Mueller T. D., Nickel J., Biomacromolecules 2017, 18, 695; - PubMed
- i) Andrew C. D., Pierluca P., Jessica N., Gráinne M. C., Daniel J. K., Biomaterials 2018, 162, 34. - PubMed
-
- Macri L., Silverstein D., Clark R. A. F., Adv. Drug Del. Rev. 2007, 59, 1366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 51621002/National Natural Science Foundation of China
- 31800801/National Natural Science Foundation of China
- 20XD1421400/Program of Shanghai Academic/Technology Research Leader
- 2021 Sci &/Shanghai Frontier Science Research Base of Optogenetic Techniques for Cell Metabolism (Shanghai Municipal Education Commission)
- Tech 03-28/Shanghai Frontier Science Research Base of Optogenetic Techniques for Cell Metabolism (Shanghai Municipal Education Commission)
LinkOut - more resources
Full Text Sources
