Analysis of the evolution, infectivity and antigenicity of circulating rabies virus strains
- PMID: 35570580
- PMCID: PMC9176641
- DOI: 10.1080/22221751.2022.2078742
Analysis of the evolution, infectivity and antigenicity of circulating rabies virus strains
Abstract
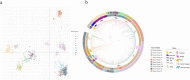
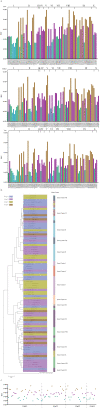
Rabies virus has existed for thousands of years and is circulating in many species. In the present study, a total of 2896 rabies viruses isolated worldwide were phylogenetically classified into ten clusters based on the G gene sequence, and these clusters showed a close relationship with the hosts and regions that they were isolated from. Eighty-three representative G sequences were selected from ten clusters and were used to construct pseudoviruses using the VSV vector. The phylogenetic relationships, infectivity and antigenicity of the representative 83 pseudotyped rabies viruses were comprehensively analyzed. Eighty three pseudoviruses were divided into four antigentic clusters (GAgV), of which GAgV4 showed poor neutralization to all immunized sera. Further analysis showed that almost all strains in the GAgV4 were isolated from wild animals in the America, especially bats and skunks. No significant relationship in terms of phylogeny, infectivity and antigenicity was proved. Amino acid mutations at residues 231and 436 can affect the infectivity, while mutations at residues 113, 164 and 254 may affect the sensitivity to immunized animal sera, especially residue 254. We recommend close monitoring of infectivity and antigenicity, which should be more precise than simple genetic analysis.
Keywords: Rabies virus; antigenic evolution; genetic evolution; infectivity.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Zhang YZ, Xiong CL, Lin XD, et al. . Genetic diversity of Chinese rabies viruses: evidence for the presence of two distinct clades in China. Infect Genet Evol J. 2009;9(1):87–96. - PubMed
-
- Leung A, Davies HD, Hon K.. Rabies: epidemiology, pathogenesis, and prophylaxis. Adv Ther. 2007;24(6):1340–1347. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
