Liver Colonization by Colorectal Cancer Metastases Requires YAP-Controlled Plasticity at the Micrometastatic Stage
- PMID: 35570706
- PMCID: PMC9381095
- DOI: 10.1158/0008-5472.CAN-21-0933
Liver Colonization by Colorectal Cancer Metastases Requires YAP-Controlled Plasticity at the Micrometastatic Stage
Abstract
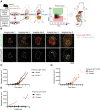
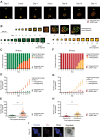
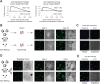
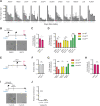
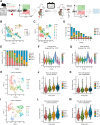
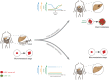
Micrometastases of colorectal cancer can remain dormant for years prior to the formation of actively growing, clinically detectable lesions (i.e., colonization). A better understanding of this step in the metastatic cascade could help improve metastasis prevention and treatment. Here we analyzed liver specimens of patients with colorectal cancer and monitored real-time metastasis formation in mouse livers using intravital microscopy to reveal that micrometastatic lesions are devoid of cancer stem cells (CSC). However, lesions that grow into overt metastases demonstrated appearance of de novo CSCs through cellular plasticity at a multicellular stage. Clonal outgrowth of patient-derived colorectal cancer organoids phenocopied the cellular and transcriptomic changes observed during in vivo metastasis formation. First, formation of mature CSCs occurred at a multicellular stage and promoted growth. Conversely, failure of immature CSCs to generate more differentiated cells arrested growth, implying that cellular heterogeneity is required for continuous growth. Second, early-stage YAP activity was required for the survival of organoid-forming cells. However, subsequent attenuation of early-stage YAP activity was essential to allow for the formation of cell type heterogeneity, while persistent YAP signaling locked micro-organoids in a cellularly homogenous and growth-stalled state. Analysis of metastasis formation in mouse livers using single-cell RNA sequencing confirmed the transient presence of early-stage YAP activity, followed by emergence of CSC and non-CSC phenotypes, irrespective of the initial phenotype of the metastatic cell of origin. Thus, establishment of cellular heterogeneity after an initial YAP-controlled outgrowth phase marks the transition to continuously growing macrometastases.
Significance: Characterization of the cell type dynamics, composition, and transcriptome of early colorectal cancer liver metastases reveals that failure to establish cellular heterogeneity through YAP-controlled epithelial self-organization prohibits the outgrowth of micrometastases. See related commentary by LeBleu, p. 1870.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
- Cancer Res. 82:1870.
- Cancer Res. 82:1870.
References
-
- Schepers AG, Snippert HJ, Stange DE, Van Den Born M, Van Es JH, Van De Wetering M, et al. . Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 2012;337:730–5. - PubMed
-
- Shimokawa M, Ohta Y, Nishikori S, Matano M, Takano A, Fujii M, et al. . Visualization and targeting of LGR5 + human colon cancer stem cells. Nature 2017;545:187–92. - PubMed
-
- De Sousa E Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, et al. . A distinct role for Lgr5 + stem cells in primary and metastatic colon cancer. Nature 2017;543:676–80. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

