Cascade Alternating Metathesis Cyclopolymerization of Diynes and Dihydrofuran
- PMID: 35570817
- PMCID: PMC9173669
- DOI: 10.1021/acsmacrolett.2c00140
Cascade Alternating Metathesis Cyclopolymerization of Diynes and Dihydrofuran
Abstract
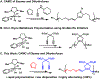
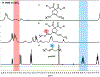
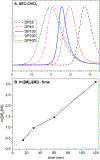
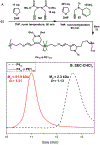
Ruthenium alkoxymethylidene complexes have recently come into view as competent species for metathesis copolymerization reactions when coupled with appropriate comonomer targets. Here, we explore the ability of Fischer-type carbenes to participate in cascade alternating metathesis cyclopolymerization (CAMC) through facile terminal alkyne addition. The combination of diyne monomers and an equal feed ratio of low-strain dihydrofuran leads to a controlled chain-growth copolymerization with high degrees of alternation (>97% alternating diads) and produces degradable polymer materials with low dispersities and targetable molecular weights. When combined with enyne monomers, this method is amenable to the synthesis of alternating diblock copolymers that can be fully degraded to short oligomer fragments under aqueous acidic conditions. This work furthers the potential for the generation of functional metathesis materials via Fischer-type ruthenium alkylidenes.
Figures
Similar articles
-
Alternating Cascade Metathesis Polymerization of Enynes and Cyclic Enol Ethers with Active Ruthenium Fischer Carbenes.J Am Chem Soc. 2020 Jul 29;142(30):12942-12947. doi: 10.1021/jacs.0c06045. Epub 2020 Jul 20. J Am Chem Soc. 2020. PMID: 32662989 Free PMC article.
-
Living Alternating Ring-Opening Metathesis Copolymerization of 2,3-Dihydrofuran to Provide Completely Degradable Polymers.Angew Chem Int Ed Engl. 2023 Nov 20;62(47):e202309632. doi: 10.1002/anie.202309632. Epub 2023 Oct 17. Angew Chem Int Ed Engl. 2023. PMID: 37789610
-
Controlled Alternating Metathesis Copolymerization of Terminal Alkynes.ACS Macro Lett. 2022 Jul 19;11(7):847-853. doi: 10.1021/acsmacrolett.2c00258. Epub 2022 Jun 23. ACS Macro Lett. 2022. PMID: 35736023
-
Functional Precision Polymers via Stereo- and Regioselective Polymerization Using Group 6 Metal Alkylidene and Group 6 and 8 Metal Alkylidene N-Heterocyclic Carbene Complexes.Macromol Rapid Commun. 2019 Jan;40(1):e1800492. doi: 10.1002/marc.201800492. Epub 2018 Aug 17. Macromol Rapid Commun. 2019. PMID: 30118168 Review.
-
Well-Defined Alkyne Metathesis Catalysts: Developments and Recent Applications.Chemistry. 2019 Mar 1;25(13):3190-3208. doi: 10.1002/chem.201804511. Epub 2018 Dec 14. Chemistry. 2019. PMID: 30346054 Review.
Cited by
-
Highly Strained Tricyclic Oxanorbornenes with Uncommon Reactivity Enable Rapid ROMP for Thermally High-Performing Polyenes.Macromolecules. 2025 Apr 4;58(8):4215-4224. doi: 10.1021/acs.macromol.4c02601. eCollection 2025 Apr 22. Macromolecules. 2025. PMID: 40290572 Free PMC article.
References
-
- Sutthasupa S; Shiotsuki M; Sanda F Recent Advances in Ring-Opening Metathesis Polymerization, and Application to Synthesis of Functional Materials. Polym. J 2010, 42, 905–915.
-
- Leitgeb A; Wappel J; Slugovc C The ROMP Toolbox Upgraded. Polymer. 2010, 51, 2927–2946.
-
- Kang EH; Lee IS; Choi TL Ultrafast Cyclopolymerization for Polyene Synthesis: Living Polymerization to Dendronized Polymers. J. Am. Chem. Soc 2011, 133, 11904–11907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

