Myrtenal mitigates streptozotocin-induced spatial memory deficit via improving oxido inflammatory, cholinergic and neurotransmitter functions in mice
- PMID: 35570857
- PMCID: PMC9095925
- DOI: 10.1016/j.crphar.2022.100106
Myrtenal mitigates streptozotocin-induced spatial memory deficit via improving oxido inflammatory, cholinergic and neurotransmitter functions in mice
Abstract
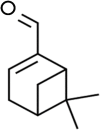
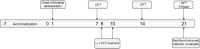
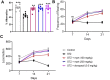
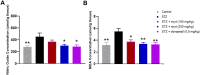
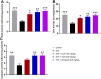
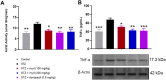
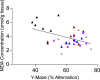
The occurrence of chronic neurodegenerative disorders is on the rise, but with no effective treatment due to the paucity of information on the pathological mechanism underlying these disorders. Thus, this study investigated the role of oral administration of myrtenal in mitigating memory deficits and neuro-biochemical alterations in streptozotocin-demented mice model. Mice (n = 35) were randomly allocated into five cohorts consisting of 7 mice each; Group I: Control mice received vehicle alone; Group II: streptozotocin; Group III: streptozotocin + 100 mg/kg myrtenal; Group IV: streptozotocin +200 mg/kg myrtenal; and Group V: streptozotocin + donepezil 0.5 mg/kg. Data from this study demonstrated that the administration of streptozotocin (STZ) impaired spatial memory and induced alterations in markers of oxido-inflammatory response, cholinergic function, cytoarchitecture, and neurotransmitter levels in mice hippocampus. Notably, administration of myrtenal enhanced spatial memory performance in STZ-demented mice by improving the activities of endogenous antioxidant enzymes to protect the brain from oxido-inflammatory stress. Treatment with myrtenal also restored cholinergic function and stabilized the homeostasis of neurotransmitters in STZ-demented mice. The authors infer that fruits rich in myrtenal may be beneficial for treating patients living with dementia associated with Alzheimer's disease.
Keywords: Antioxidant; Dementia; Memory; Mice; Myrtenal; Streptozotocin.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Myrtenal improves memory deficits in mice exposed to radiofrequency-electromagnetic radiation during gestational and neonatal development via enhancing oxido-inflammatory, and neurotransmitter functions.Heliyon. 2023 Apr 8;9(4):e15321. doi: 10.1016/j.heliyon.2023.e15321. eCollection 2023 Apr. Heliyon. 2023. PMID: 37123912 Free PMC article.
-
Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular streptozotocin induced experimental dementia in rats.Biomed Pharmacother. 2016 Jul;81:56-62. doi: 10.1016/j.biopha.2016.03.017. Epub 2016 Apr 9. Biomed Pharmacother. 2016. PMID: 27261577
-
Neuroprotective Effects of Myrtenal in an Experimental Model of Dementia Induced in Rats.Antioxidants (Basel). 2022 Feb 12;11(2):374. doi: 10.3390/antiox11020374. Antioxidants (Basel). 2022. PMID: 35204256 Free PMC article.
-
Moringa oleifera-supplemented diet protect against cortico-hippocampal neuronal degeneration in scopolamine-induced spatial memory deficit in mice: role of oxido-inflammatory and cholinergic neurotransmission pathway.Metab Brain Dis. 2021 Dec;36(8):2445-2460. doi: 10.1007/s11011-021-00855-9. Epub 2021 Oct 20. Metab Brain Dis. 2021. PMID: 34669098
-
Therapeutic Potential of Myrtenal and Its Derivatives-A Review.Life (Basel). 2023 Oct 20;13(10):2086. doi: 10.3390/life13102086. Life (Basel). 2023. PMID: 37895468 Free PMC article. Review.
Cited by
-
Human placental extract rescues hippocampal damage associated with cognitive impairment in diabetic male rats through antioxidative, anti-inflammatory, and neuromodulatory activities.Metab Brain Dis. 2025 Jun 16;40(6):225. doi: 10.1007/s11011-025-01646-2. Metab Brain Dis. 2025. PMID: 40522516 Free PMC article.
-
Myrtenal improves memory deficits in mice exposed to radiofrequency-electromagnetic radiation during gestational and neonatal development via enhancing oxido-inflammatory, and neurotransmitter functions.Heliyon. 2023 Apr 8;9(4):e15321. doi: 10.1016/j.heliyon.2023.e15321. eCollection 2023 Apr. Heliyon. 2023. PMID: 37123912 Free PMC article.
-
Nanoformulated 3'-diindolylmethane modulates apoptosis, migration, and angiogenesis in breast cancer cells.Heliyon. 2023 Dec 10;10(1):e23553. doi: 10.1016/j.heliyon.2023.e23553. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38187226 Free PMC article.
-
Ameliorating the Role of Aripiprazole in Memory Deficits Induced by Intracerebroventricular Streptozotocin-Induced Dementia of Alzheimer's Type.ACS Omega. 2023 Jul 6;8(28):25295-25302. doi: 10.1021/acsomega.3c02550. eCollection 2023 Jul 18. ACS Omega. 2023. Retraction in: ACS Omega. 2024 Mar 21;9(13):15726. doi: 10.1021/acsomega.4c02182. PMID: 37483219 Free PMC article. Retracted.
-
Myrtenal Pretreatment Exerts a Protective Effect Against Renal Ischemia-Reperfusion Injury in Rats.J Biochem Mol Toxicol. 2025 Mar;39(3):e70202. doi: 10.1002/jbt.70202. J Biochem Mol Toxicol. 2025. PMID: 40025781 Free PMC article.
References
-
- Alvarez E.O., Beauquis J., Revsin Y., Banzan A.M., Roig P., De Nicola A.F., Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. 2009;198(1):224–230. - PubMed
-
- Begum S., Ali M., Gul H., Ahmad W., Alam S., Khan M., et al. In vitro enzyme inhibition activities of Myrtus communis L. Afr. J. Pharm. Pharmacol. 2012;6:1083–1087.
-
- Biessels G.-J., Kamal A., Urban I.J., Spruijt B.M., Erkelens D.W., Gispen W.H. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800(1):125–135. - PubMed
LinkOut - more resources
Full Text Sources

