Pulmonary arteriovenous malformation and inherent complications with solitary lung nodule biopsy-literature overview and case report
- PMID: 35570867
- PMCID: PMC9096463
- DOI: 10.1016/j.radcr.2022.04.003
Pulmonary arteriovenous malformation and inherent complications with solitary lung nodule biopsy-literature overview and case report
Abstract
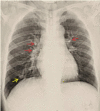
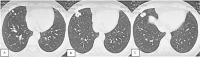
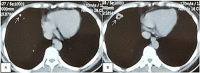
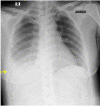
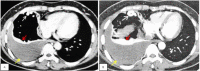
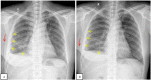
Pulmonary arteriovenous malformation, also known as an arteriovenous fistula, is typically a congenital disease caused by structural deficiencies, particularly the lack of capillary wall development, leading to the abnormal dilation of the pulmonary capillaries. The majority of pulmonary arteriovenous malformation cases are associated with Rendu-Osler-Weber syndrome, also known as hereditary hemorrhagic telangiectasia. Pulmonary arteriovenous malformation rarely occurs due to chest trauma. Pulmonary arteriovenous malformations are long-lasting and often first diagnosed in adults. More than two-thirds of pulmonary arteriovenous malformation lesions are found in the lower lung lobe and the subpleural area, and the vast majority of cases present with the monofocal form. The initial diagnosis is often based on the identification of a solitary pulmonary nodule. However, a solitary nodule detected on chest computed tomography that is not correctly diagnosed as pulmonary arteriovenous malformation, even after intravenous contrast injection, can lead to the performance of a transthoracic biopsy. Biopsy of pulmonary arteriovenous malformations can lead to stroke occurrence, during which the patient often presents with severe pleural bleeding, which can have lifelong consequences if not immediately treated. We report a case of pulmonary arteriovenous malformation that was discovered incidentally in an adult patient who underwent non-contrast computed tomography. Misdiagnosis occurred, and transthoracic lung biopsy was performed. Complications were discovered late, and the patient underwent surgical pulmonary arteriovenous malformation removal and was treated for hemothorax.
Keywords: Hemothorax; Hereditary hemorrhagic Telangiectasia; Pulmonary arteriovenous malformation; Rare diseases; Vascular malformation.
© 2022 The Authors. Published by Elsevier Inc. on behalf of University of Washington.
Figures


References
-
- Richard Webb W, Higgins CB. Thoracic imaging: pulmonary and cardiovascular radiology, Wolters KluwerPhiladelphia, The United States of America, 2017; 3 E; p. 275–305.
-
- Gungor S, Yakar HI. Usefulness of dynamic fluorodeoxyglucose positron emission tomography/computed tomography in diagnosing pulmonary arteriovenous malformation mimicking a lung tumour. Interact Cardiovasc Thorac Surg. 2021;33(4):665–667. doi: 10.1093/icvts/ivab130. PMID: 33954790. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

