Curcumin Restrains Oxidative Stress of After Intracerebral Hemorrhage in Rat by Activating the Nrf2/HO-1 Pathway
- PMID: 35571134
- PMCID: PMC9092178
- DOI: 10.3389/fphar.2022.889226
Curcumin Restrains Oxidative Stress of After Intracerebral Hemorrhage in Rat by Activating the Nrf2/HO-1 Pathway
Abstract
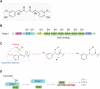
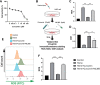
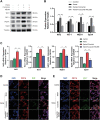
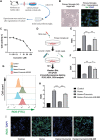
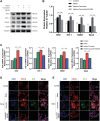
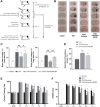
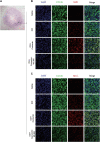
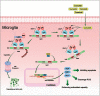
Intracerebral hemorrhage (ICH), a severe hemorrhagic stroke, induces cerebral oxidative stress and severe secondary neurological injury. Curcumin was demonstrated to inhibit oxidative stress in the brain after ICH. However, the pharmacological mechanism needs further research. We used an intrastriatal injection of autologous blood to make the rat ICH model, and then the rat was treated with curcumin (100 mg/kg/day). Modified Neurological Severity Score (mNSS) and corner test results showed that curcumin could significantly promote the neurological recovery of ICH rats. Meanwhile, curcumin could substantially reduce ROS and MDA in the tissues around intracranial hematoma and prevent GSH depletion. To explore the pharmacological molecular mechanism of curcumin, we used HAPI cells and primary rat cortical microglia for in vitro experiments. In vitro, heme-treated cells were used as the cell model of ICH to explore the molecular mechanism of inhibiting oxidative stress by curcumin treatment. The results showed that curcumin significantly inhibited heme-induced oxidative stress, decreased intracellular ROS and MDA, and promoted Nrf2 and its downstream antioxidant gene (HO-1, NQO1, and Gpx4) expression. These results suggest that curcumin inhibits oxidative stress by activating the Nrf2/HO-1 pathway. Here, our results indicate that curcumin can promote the inhibition of oxidative stress in microglia by activating the Nrf2/HO-1 pathway and promoting neurological recovery after ICH, providing a new therapeutic target for clinical treatment of ICH.
Keywords: HO-1; Nrf2; ROS; curcumin; hematoma; intracerebral hemorrhage; microglia; oxidative stress.
Copyright © 2022 Duan, Wang, Jiao, Geng, Wu, Yan and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

