CAPRIN1 Enhances Chemoresistance and Glycolysis in Laryngeal Squamous Cell Carcinoma via Regulation of ZIC5
- PMID: 35571493
- PMCID: PMC9098336
- DOI: 10.1155/2022/6160539
CAPRIN1 Enhances Chemoresistance and Glycolysis in Laryngeal Squamous Cell Carcinoma via Regulation of ZIC5
Abstract
Background: Cytoplasmic activation/proliferation-associated protein-1 (CAPRIN1) plays an important role in carcinogenesis, whereas its role in laryngeal squamous cell carcinoma remains unclear. This study was designed to investigate the roles of CAPRIN1 in glycolysis and chemoresistance and its underlying mechanisms in laryngeal squamous cell carcinoma.
Methods: Cell viability was evaluated by using CCK-8 and colony formation assays. qRT-PCR, Western blotting, and immunohistochemistry were used to determine the expressions of target genes. Gene knockdown and overexpression cell lines were constructed by performing transfection of siRNAs and plasmids, respectively. Luciferase reporter assay, RNA pull-down, and RNA immunoprecipitation assays were applied to evaluate the RNA-protein interactions. The Kaplan-Meier analysis was performed to evaluate the relationship between gene expression and overall survival rate.
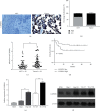
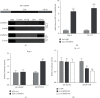
Results: An elevation of CAPRIN1 was identified to be associated with chemoresistance and poor prognosis in patients with laryngeal cancer. The increase of CAPRIN1 promoted glycolysis and chemoresistance, whereas the knockdown of CAPRIN1 inhibited glycolysis and chemoresistance in laryngeal cancer cells. The underlying mechanistic investigation revealed that CAPRIN1 promoted glycolysis and chemoresistance of laryngeal cancer cells by the regulation of Zic Family Member 5 (ZIC5).
Conclusion: CAPRIN1 promoted laryngeal squamous cell carcinoma glycolysis and chemoresistance by the regulation of ZIC5.
Copyright © 2022 Yubo Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
-
- Jurkiewicz D., Dzaman K., Rapiejko P. Laryngeal cancer risk factors. Polski Merkuriusz Lekarski: Organ Polskiego Towarzystwa Lekarskiego. . 2006;21(121):94–98. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

