De novo variants of CSNK2B cause a new intellectual disability-craniodigital syndrome by disrupting the canonical Wnt signaling pathway
- PMID: 35571680
- PMCID: PMC9092267
- DOI: 10.1016/j.xhgg.2022.100111
De novo variants of CSNK2B cause a new intellectual disability-craniodigital syndrome by disrupting the canonical Wnt signaling pathway
Abstract
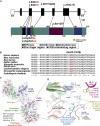
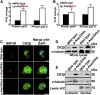
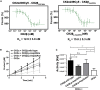
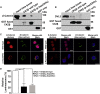
CSNK2B encodes for casein kinase II subunit beta (CK2β), the regulatory subunit of casein kinase II (CK2), which is known to mediate diverse cellular pathways. Variants in this gene have been recently identified as a cause of Poirier-Bienvenu neurodevelopmental syndrome (POBINDS), but functional evidence is sparse. Here, we report five unrelated individuals: two of them manifesting POBINDS, while three are identified to segregate a new intellectual disability-craniodigital syndrome (IDCS), distinct from POBINDS. The three IDCS individuals carried two different de novo missense variants affecting the same codon of CSNK2B. Both variants, NP_001311.3; p.Asp32His and NP_001311.3; p.Asp32Asn, lead to an upregulation of CSNK2B expression at transcript and protein level, along with global dysregulation of canonical Wnt signaling. We found impaired interaction of the two key players DVL3 and β-catenin with mutated CK2β. The variants compromise the kinase activity of CK2 as evident by a marked reduction of phosphorylated β-catenin and consequent absence of active β-catenin inside nuclei of the patient-derived lymphoblastoid cell lines (LCLs). In line with these findings, whole-transcriptome profiling of patient-derived LCLs harboring the NP_001311.3; p.Asp32His variant confirmed a marked difference in expression of genes involved in the Wnt signaling pathway. In addition, whole-phosphoproteome analysis of the LCLs of the same subject showed absence of phosphorylation for 313 putative CK2 substrates, enriched in the regulation of nuclear β-catenin and transcription of the target genes. Our findings suggest that discrete variants in CSNK2B cause dominant-negative perturbation of the canonical Wnt signaling pathway, leading to a new craniodigital syndrome distinguishable from POBINDS.
Keywords: CK2; CK2α; CK2β; CSNK2B; DVL3; GestaltMatcher; POBINDS; Wnt signaling; intellectual disability-craniodigital syndrome; whole transcriptome profiling; whole-phosphoproteome profiling; β-catenin.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hussain M.S., Battaglia A., Szczepanski S., Kaygusuz E., Toliat M.R., Yigit G., Beleggia F., Tinschert S., Clayton-Smith J., Vasudevan P., et al. Mutations in CKAP2L, the human homolog of the mouse Radmis gene, cause Filippi syndrome. Am. J. Hum. Genet. 2014;95:622–632. doi: 10.1016/j.ajhg.2014.10.008. - DOI - PMC - PubMed
-
- Balasubramanian M., Lord H., Levesque S., Guturu H., Thuriot F., Sillon G., Bowen J., Calhoun A.R., Viskochil D.H., Bejerano G., et al. DDD Study Chitayat syndrome: hyperphalangism, characteristic facies, hallux valgus and bronchomalacia results from a recurrent c. 266A> G p.(Tyr89Cys) variant in the ERF gene. J. Med. Genet. 2017;54:157–165. doi: 10.1136/jmedgenet-2016-104143. - DOI - PubMed
-
- Kaygusuz E., Khayyat A.I.A., Abdullah U., Budde B.S., Asif M., Ahmed I., Makhdoom E.U.H., Sur Erdem I., Baig J.M., Khan M.M.A., et al. A 24 generation old founder mutation impairs splicing of RBBP8 in Pakistani families affected with Jawad syndrome. Clin. Genet. 2021;100:486–488. doi: 10.1111/cge.14028. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

