Chemical Solution Deposition of Insulating Yttria Nanolayers as Current Flow Diverter in Superconducting GdBa2Cu3O7-δ Coated Conductors
- PMID: 35571796
- PMCID: PMC9096825
- DOI: 10.1021/acsomega.1c05352
Chemical Solution Deposition of Insulating Yttria Nanolayers as Current Flow Diverter in Superconducting GdBa2Cu3O7-δ Coated Conductors
Abstract
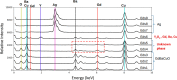
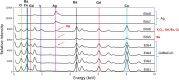
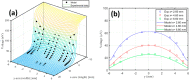
The primary benefit of a metallic stabilization/shunt in high temperature superconductor (HTS) coated conductors (CCs) is to prevent joule heating damage by providing an alternative path for the current flow during the HTS normal state transition (i.e., quench). However, the shunt presence in combination with unavoidable fluctuations in the critical current (I c) of the HTS film can develop a localized quench along the CC's length if the operational current is kept close to I c. This scenario, also known as the hot-spot regime, can lead to the rupture of the CC if the local quench does not propagate fast enough. The current flow diverter (CFD) is the CC architecture concept that has proven to increase the conductor's robustness against a hot-spot regime by simply boosting the quench velocity in the CC, which avoids the shunt compromise in some applications. This work investigates a practical manufacturing route for incorporating the CFD architecture in a reel-to-reel system via the preparation of yttrium oxide (Y2O3) as an insulating thin nanolayer (∼100 nm) on top of a GdBa2Cu3O7 (GdBCO) superconductor. Chemical solution deposition (CSD) using ink jet printing (IJP) is shown to be a suitable manufacturing approach. Two sequences of the experimental steps have been investigated, where oxygenation of the GdBCO layer is performed after or before the solution deposition and the Y2O3 nanolayer thermal treatment formation step. A correlated analysis of the microstructure, in situ oxygenation kinetics, and superconducting properties of the Ag/Y2O3/GdBCO trilayer processed under different conditions shows that a new customized functional CC can be prepared. The successful achievement of the CFD effect in the case of the preoxygenated customized CC was confirmed by measuring the current transfer length, thus demonstrating the effectiveness of the CSD-IJP as a processing method.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Obradors X.; Puig T. Coated Conductors for Power Applications: Materials Challenges. Supercond. Sci. Technol. 2014, 27 (4), 044003.10.1088/0953-2048/27/4/044003. - DOI
-
- MacManus-Driscoll J. L.; Wimbush S. C. Processing and Application of High-Temperature Superconducting Coated Conductors. Nat. Rev. Mater. 2021, 6 (7), 587–604. 10.1038/s41578-021-00290-3. - DOI
-
- Awaji S.; Watanabe K.; Oguro H.; Miyazaki H.; Hanai S.; Tosaka T.; Ioka S. First Performance Test of a 25 T Cryogen-Free Superconducting Magnet. Supercond. Sci. Technol. 2017, 30 (6), 065001.10.1088/1361-6668/aa6676. - DOI
-
- Tixador P.Superconducting Fault Current Limiter; World Scientific Series in Applications of Superconductivity and Related Phenomena; World Scientific, 2018; Vol. 03. 10.1142/11062. - DOI
-
- Tixador P.; Bauer M.; Bruzek C.-E.; Calleja A.; Deutscher G.; Dutoit B.; Gomory F.; Martini L.; Noe M.; Obradors X.; Pekarcikova M.; Sirois F. Status of the European Union Project FASTGRID. IEEE Trans. Appl. Supercond. 2019, 29 (5), 1–5. 10.1109/TASC.2019.2908586. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

