RSV Prevention in All Infants: Which Is the Most Preferable Strategy?
- PMID: 35572550
- PMCID: PMC9096079
- DOI: 10.3389/fimmu.2022.880368
RSV Prevention in All Infants: Which Is the Most Preferable Strategy?
Abstract
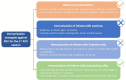
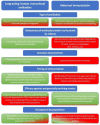
Respiratory syncytial virus (RSV) causes a spectrum of respiratory illnesses in infants and young children that may lead to hospitalizations and a substantial number of outpatient visits, which result in a huge economic and healthcare burden. Most hospitalizations happen in otherwise healthy infants, highlighting the need to protect all infants against RSV. Moreover, there is evidence on the association between early-life RSV respiratory illness and recurrent wheezing/asthma-like symptoms As such, RSV is considered a global health priority. However, despite this, the only prevention strategy currently available is palivizumab, a monoclonal antibody (mAb) indicated in a subset of preterm infants or those with comorbidities, hence leaving the majority of the infant population unprotected against this virus. Therefore, development of prevention strategies against RSV for all infants entering their first RSV season constitutes a large unmet medical need. The aim of this review is to explore different immunization approaches to protect all infants against RSV. Prevention strategies include maternal immunization, immunization of infants with vaccines, immunization of infants with licensed mAbs (palivizumab), and immunization of infants with long-acting mAbs (e.g., nirsevimab, MK-1654). Of these, palivizumab use is restricted to a small population of infants and does not offer a solution for all-infant protection, whereas vaccine development in infants has encountered various challenges, including the immaturity of the infant immune system, highlighting that future pediatric vaccines will most likely be used in older infants (>6 months of age) and children. Consequently, maternal immunization and immunization of infants with long-acting mAbs represent the two feasible strategies for protection of all infants against RSV. Here, we present considerations regarding these two strategies covering key areas which include mechanism of action, "consistency" of protection, RSV variability, duration of protection, flexibility and optimal timing of immunization, benefit for the mother, programmatic implementation, and acceptance of each strategy by key stakeholders. We conclude that, based on current data, immunization of infants with long-acting mAbs might represent the most effective approach for protecting all infants entering their first RSV season.
Keywords: RSV; asthma; lower respiratory tract infection; maternal immunization; monoclonal antibody; nirsevimab; palivizumab; prevention.
Copyright © 2022 Esposito, Abu Raya, Baraldi, Flanagan, Martinon Torres, Tsolia and Zielen.
Conflict of interest statement
SE: Speaker’s fees from GSK, Pfizer, Novartis, Sanofi Pasteur, MSD and Vifor in the past three years. BA is supported by the Michael Smith Foundation for Health Research and received payment as a scientific expert of the Sanofi group of companies. KF is a member of the Australian Technical Advisory Group on Immunisation noting that this paper represents her own personal view; received honoraria as a member of the vaccine advisory boards for Seqiris and Sanofi Pasteur in the last 5 years. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. . Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet (2017) 390(10098):946–58. doi: 10.1016/S0140-6736(17)30938-8 - DOI - PMC - PubMed
-
- Plotkin SA OW, Offitt PA, Edwards KMRK. Respiratory Syncytial Virus Vaccines. Philadelphia (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

