Virological and Clinical Determinants of the Magnitude of Humoral Responses to SARS-CoV-2 in Mild-Symptomatic Individuals
- PMID: 35572570
- PMCID: PMC9097229
- DOI: 10.3389/fimmu.2022.860215
Virological and Clinical Determinants of the Magnitude of Humoral Responses to SARS-CoV-2 in Mild-Symptomatic Individuals
Abstract
Background: Evidence on the determinants of the magnitude of humoral responses and neutralizing titers in individuals with mild COVID-19 is scarce.
Methods: In this cohort study of mild COVID-19 patients, we assessed viral load (VL) by RT-qPCR at two/three time points during acute infection, and anti-SARS-CoV-2 antibodies by ELISA and plasma neutralizing responses using a pseudovirus assay at day 60.
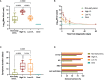
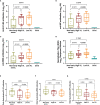
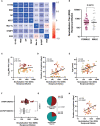
Results: Seventy-one individuals (65% female, median 42 years old) were recruited and grouped into high viral load (VL) >7.5 Log10 copies/mL (n=20), low, VL ≤7.5 Log10 copies/mL (n=22), or as Non-early seroconverters with a positive PCR (n=20), and healthy individuals with a negative PCR (n=9). Individuals with high or low VL showed similar titers of total neutralizing antibodies at day 60, irrespective of maximal VL or viral dynamics. Non-early seroconverters had lower antibody titers on day 60, albeit similar neutralizing activity as the groups with high or low VL. Longer symptom duration and older age were independently associated with increased humoral responses.
Conclusions: In mild SARS-CoV-2-infected individuals, the duration of symptoms and age (but not VL) contribute to higher humoral responses.
Keywords: COVID-19; humoral response; neutralizing antibodies; seroconversion; symptoms; viral load.
Copyright © 2022 Pradenas, Ubals, Urrea, Suñer, Trinité, Riveira-Muñoz, Marfil, Ávila-Nieto, Rodríguez de la Concepción, Tarrés-Freixas, Laporte, Ballana, Carrillo, Clotet, Mitjà and Blanco.
Conflict of interest statement
Outside the submitted work JB and JC are founders and shareholders of AlbaJuna Therapeutics, S.L. BC is founder and shareholder of AlbaJuna Therapeutics, S.L and AELIX Therapeutics, S.L. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Carrillo J, Izquierdo-Useros N, Ávila-Nieto C, Pradenas E, Clotet B, Blanco J. Humoral Immune Responses and Neutralizing Antibodies Against SARS-CoV-2; Implications in Pathogenesis and Protective Immunity. Biochem Biophys Res Commun (2021) 538:187–91. doi: 10.1016/j.bbrc.2020.10.108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

