Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects
- PMID: 35572592
- PMCID: PMC9092649
- DOI: 10.3389/fimmu.2022.843928
Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects
Abstract
The persistent coronavirus disease 2019 (COVID-19), characterized by severe respiratory syndrome, is caused by coronavirus 2 (SARS-CoV-2), and it poses a major threat to public health all over the world. Currently, optimal COVID-19 management involves effective vaccination. Vaccination is known to greatly enhance immune response against viral infections and reduce public transmission of COVID-19. However, although current vaccines offer some benefits, viral variations and other factors demand the continuous development of vaccines to eliminate this virus from host. Hence, vaccine research and development is crucial and urgent to the elimination of this pandemic. Herein, we summarized the structural and replicatory features of SARS-CoV-2, and focused on vaccine-mediated disease prevention strategies like vaccine antigen selection, vaccine research, and vaccine application. We also evaluated the latest literature on COVID-19 and extensively reviewed action mechanisms, clinical trial (CT) progresses, advantages, as well as disadvantages of various vaccine candidates against SARS-CoV-2. Lastly, we discussed the current viral treatment, prevention trends, and future prospects.
Keywords: COVID-19; COVID-19 vaccines; SARS-CoV-2; antigen selection; prevention.
Copyright © 2022 Zhang, Shen and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

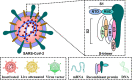
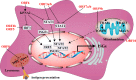
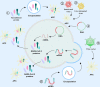


References
-
- COVID-19 - China . WHO Disease Outbreak News (2020). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229.
-
- Novel Coronavirus (2019-nCoV) . WHO Situation Report-1 (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio....
-
- WHO Technical Guidance . Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica....
-
- WHO Coronavirus (COVID-19) Dashboard . WHO Global Situation (2021). Available at: https://covid19.who.int/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

