Probiotics for the Management of Infectious Diseases: Reviewing the State of the Art
- PMID: 35572661
- PMCID: PMC9096241
- DOI: 10.3389/fmicb.2022.877142
Probiotics for the Management of Infectious Diseases: Reviewing the State of the Art
Abstract
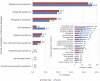
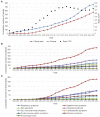
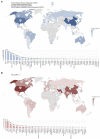
This review aims to provide insight into the potential of probiotics as a clinical modality targeted at infectious diseases by creating a comprehensive overview of the state of the art of research and development efforts as shown by patents and clinical trials of the past 20 years. Data were retrieved from patent and clinical trial databases to reflect the long- and short-term developments of probiotics research. The data were analyzed to extract information on the total number of patents and trials for each indication, application date and location, and applicant/sponsor type. A total of 80 infectious diseases were investigated, precipitating in 789 patents and 602 clinical trials for 67 indications studied as targets of probiotics. An increasing trend was seen for the number of patents and clinical trials that were applied for since 1999 with the highest number of patents and clinical trials targeted to digestive tract, respiratory, and urogenital indications. Overall, research demonstrated a substantial interest in probiotics targeting infectious diseases, which was in line with reported unmet needs and global probiotics sales estimates. However, the declining rate of translation from patents to clinical trials indicates that there are some barriers obstructing the research process.
Keywords: clinical trials; infectious diseases; patents; probiotics; state of the art.
Copyright © 2022 Wiegers, van de Burgwal and Larsen.
Conflict of interest statement
LB is a consultant for several commercial parties in the field of probiotics and life sciences; none of her advising practices are related to or in conflict with the content of this research. OL is Senior Manager Science at Yakult Nederland B.V. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Reviewing the state of the art of probiotics as clinical modalities for brain-gut-microbiota axis associated disorders.Front Microbiol. 2022 Nov 25;13:1053958. doi: 10.3389/fmicb.2022.1053958. eCollection 2022. Front Microbiol. 2022. PMID: 36504794 Free PMC article. Review.
-
Efaproxiral: GSJ 61, JP 4, KDD 86, RS 4, RSR 13.Drugs R D. 2005;6(3):178-85. doi: 10.2165/00126839-200506030-00007. Drugs R D. 2005. PMID: 15869322 Review.
-
Probiotics in Curing Allergic and Inflammatory Conditions - Research Progress and Futuristic Vision.Recent Pat Inflamm Allergy Drug Discov. 2017;10(2):105-118. doi: 10.2174/1872213X10666161226162229. Recent Pat Inflamm Allergy Drug Discov. 2017. PMID: 28029082 Review.
-
Probiotics as a Tool to Biosynthesize Metallic Nanoparticles: Research Reports and Patents Survey.Recent Pat Drug Deliv Formul. 2017;11(1):5-18. doi: 10.2174/1872211311666170313124335. Recent Pat Drug Deliv Formul. 2017. PMID: 28294074 Review.
-
TECHNOLOGICAL INFORMATION REGARDING PREBIOTICS AND PROBIOTICS NUTRITION VERSUS THE PATENT REGISTERS: WHAT IS NEW?Arq Bras Cir Dig. 2016 Nov-Dec;29(4):279-281. doi: 10.1590/0102-6720201600040016. Arq Bras Cir Dig. 2016. PMID: 28076487 Free PMC article.
Cited by
-
Microbiome Dynamics: A Paradigm Shift in Combatting Infectious Diseases.J Pers Med. 2024 Feb 18;14(2):217. doi: 10.3390/jpm14020217. J Pers Med. 2024. PMID: 38392650 Free PMC article. Review.
-
Concomitant Therapy of Inactivated Enterococcus faecalis CECT7121 with Fluoroquinolones in a Salmonella Enteritidis Murine Sepsis Model.Indian J Microbiol. 2024 Dec;64(4):1956-1960. doi: 10.1007/s12088-024-01192-y. Epub 2024 Feb 20. Indian J Microbiol. 2024. PMID: 39678964
-
Music exposure enhances resistance to Salmonella infection by promoting healthy gut microbiota.Microbiol Spectr. 2025 Mar 25;13(5):e0237724. doi: 10.1128/spectrum.02377-24. Online ahead of print. Microbiol Spectr. 2025. PMID: 40130867 Free PMC article.
-
Reviewing the state of the art of probiotics as clinical modalities for brain-gut-microbiota axis associated disorders.Front Microbiol. 2022 Nov 25;13:1053958. doi: 10.3389/fmicb.2022.1053958. eCollection 2022. Front Microbiol. 2022. PMID: 36504794 Free PMC article. Review.
-
Can Gut Microbiota Analysis Reveal Clostridioides difficile Infection? Evidence from an Italian Cohort at Disease Onset.Microorganisms. 2024 Dec 25;13(1):16. doi: 10.3390/microorganisms13010016. Microorganisms. 2024. PMID: 39858784 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

