Stress-induced increase of monoclonal antibody production in CHO cells
- PMID: 35573136
- PMCID: PMC9077828
- DOI: 10.1002/elsc.202100062
Stress-induced increase of monoclonal antibody production in CHO cells
Abstract
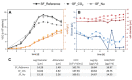
Monoclonal antibodies (mAbs) are of great interest to the biopharmaceutical industry due to their widely used application as human therapeutic and diagnostic agents. As such, mAb require to exhibit human-like glycolization patterns. Therefore, recombinant Chinese hamster ovary (CHO) cells are the favored production organisms; many relevant biopharmaceuticals are already produced by this cell type. To optimize the mAb yield in CHO DG44 cells a corelation between stress-induced cell size expansion and increased specific productivity was investigated. CO2 and macronutrient supply of the cells during a 12-day fed-batch cultivation process were tested as stress factors. Shake flasks (500 mL) and a small-scale bioreactor system (15 mL) were used for the cultivation experiments and compared in terms of their effect on cell diameter, integral viable cell concentration (IVCC), and cell-specific productivity. The achieved stress-induced increase in cell-specific productivity of up to 94.94.9%-134.4% correlates to a cell diameter shift of up to 7.34 μm. The highest final product titer of 4 g/L was reached by glucose oversupply during the batch phase of the process.
Keywords: CHO; cell size; cell‐specific productivity; mAb; stress.
© 2022 The Authors. Engineering in Life Sciences published by Wiley‐VCH GmbH.
Conflict of interest statement
We confirm that all corresponding authors agree with the submission and publication of this paper and that there is no conflict of interest concerning financial and personal relationships. The manuscript does not contain neither experiments using animals nor human studies. Furthermore, we confirm that the article has not been published previously by any of the authors and is not under consideration for publication elsewhere at the time of submission.
Figures




References
-
- Moyle D. Biomanufacturing Technology Roadmap‐Modular and Mobile, Tech. rep. BioPhorum Operations Group Ltd; 2017.
-
- Kim JY, Kim Y‐G, Lee GM. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol. 2012;93(3):917–930. - PubMed
-
- Karthik P Jayapal, Katie F Wlaschin, Wei Shou Hu, et al. Recombinant protein therapeutics from CHO Cells ‐ 20 years and counting. Chemical Engineering Progress. 2007;103(10):40–47.
-
- Freimark D, Jèrôme V, Freitag R. A GFP‐based method facilitates clonal selection of transfected CHO cells. Biotechnol J. 2010;5(1):24–31. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
