Impairment of Mitochondrial ATP Synthesis Induces RIPK3-dependent Necroptosis in Lung Epithelial Cells During Lung Injury by Lung Inflammation
- PMID: 35573150
- PMCID: PMC9066008
- DOI: 10.4110/in.2022.22.e18
Impairment of Mitochondrial ATP Synthesis Induces RIPK3-dependent Necroptosis in Lung Epithelial Cells During Lung Injury by Lung Inflammation
Abstract
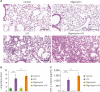
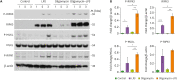
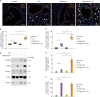
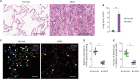
Dysfunction of mitochondrial metabolism is implicated in cellular injury and cell death. While mitochondrial dysfunction is associated with lung injury by lung inflammation, the mechanism by which the impairment of mitochondrial ATP synthesis regulates necroptosis during acute lung injury (ALI) by lung inflammation is unclear. Here, we showed that the impairment of mitochondrial ATP synthesis induces receptor interacting serine/threonine kinase 3 (RIPK3)-dependent necroptosis during lung injury by lung inflammation. We found that the impairment of mitochondrial ATP synthesis by oligomycin, an inhibitor of ATP synthase, resulted in increased lung injury and RIPK3 levels in lung tissues during lung inflammation by LPS in mice. The elevated RIPK3 and RIPK3 phosphorylation levels by oligomycin resulted in high mixed lineage kinase domain-like (MLKL) phosphorylation, the terminal molecule in necroptotic cell death pathway, in lung epithelial cells during lung inflammation. Moreover, the levels of protein in bronchoalveolar lavage fluid (BALF) were increased by the activation of necroptosis via oligomycin during lung inflammation. Furthermore, the levels of ATP5A, a catalytic subunit of the mitochondrial ATP synthase complex for ATP synthesis, were reduced in lung epithelial cells of lung tissues from patients with acute respiratory distress syndrome (ARDS), the most severe form of ALI. The levels of RIPK3, RIPK3 phosphorylation and MLKL phosphorylation were elevated in lung epithelial cells in patients with ARDS. Our results suggest that the impairment of mitochondrial ATP synthesis induces RIPK3-dependent necroptosis in lung epithelial cells during lung injury by lung inflammation.
Keywords: Acute lung injury; Lung inflammation; Mitochondrial dysfunction; Necroptosis.
Copyright © 2022. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures

References
-
- Martínez-Reyes I, Cuezva JM. The H(+)-ATP synthase: a gate to ROS-mediated cell death or cell survival. Biochim Biophys Acta. 2014;1837:1099–1112. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

