Oxidative Cyclization of 4-(2-Aminophenyl)-4-oxo-2-phenylbutanenitriles into 2-(3-Oxoindolin-2-ylidene)acetonitriles
- PMID: 35573208
- PMCID: PMC9089741
- DOI: 10.1021/acsomega.2c01238
Oxidative Cyclization of 4-(2-Aminophenyl)-4-oxo-2-phenylbutanenitriles into 2-(3-Oxoindolin-2-ylidene)acetonitriles
Abstract
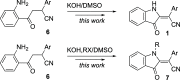
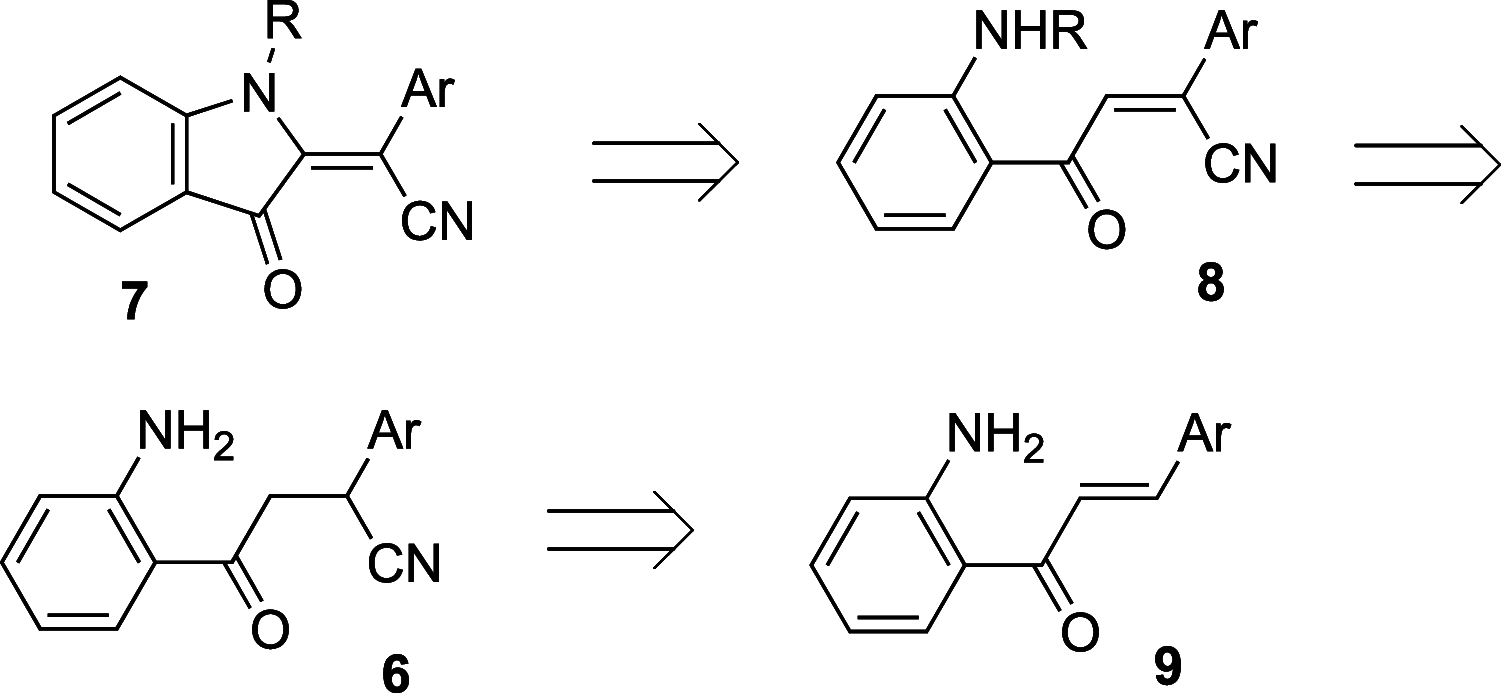
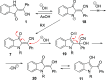
A facile and highly efficient method for the preparation of 2-(3-oxoindolin-2-ylidene)acetonitriles from 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitriles is described. The featured transformation operates via nucleophilic intramolecular cyclization and involves oxidation of the aniline moiety. Overall, this modification allowed for the improvement of yields and expansion of the reaction scope.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Cooksey C. J. Tyrian purple: 6,6′-dibromoindigo and related compounds. Molecules 2001, 6, 736–739. 10.3390/60900736. - DOI
-
- Hoessel R.; Leclerc S.; Endicott J. A.; Nobel M. E.; Lawrie A.; Tunnah P.; Leost M.; Damiens E.; Marie D.; Marko D.; Niederberger E.; Tang W.; Eisenbrand G.; Meijer L. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. 10.1038/9035. - DOI - PubMed
-
- Leclerc S.; Garnier M.; Hoessel R.; Marko D.; Bibb J. A.; Snyder G. L.; Greengard P.; Biernat J.; Wu Y.-Z.; Mandelkow E.-M.; Eisenbrand G.; Meijer L. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors?. J. Biol. Chem. 2001, 276, 251–260. 10.1074/jbc.M002466200. - DOI - PubMed
-
- Meijer L.; Skaltsounis A.-L.; Magiatis P.; Polychronopoulos P.; Knockaert M.; Leost M.; Ryan X. P.; Vonica C. A.; Brivanlou A.; Dajani R.; Crovace C.; Tarricone C.; Musacchio A.; Roe S. M.; Pearl L.; Greengard P. GSK-3-Selective Inhibitors Derived from Tyrian Purple Indirubins. Chem. Biol. 2003, 10, 1255–1266. 10.1016/j.chembiol.2003.11.010. - DOI - PubMed
-
- Polychronopoulos P.; Magiatis P.; Skaltsounis A.-L.; Myrianthopoulos V.; Mikros E.; Tarricone A.; Musacchio A.; Roe S. M.; Pearl L.; Leost M.; Greengard P.; Meijer L. Structural Basis for the Synthesis of Indirubins as Potent and Selective Inhibitors of Glycogen Synthase Kinase-3 and Cyclin-Dependent Kinases. J. Med. Chem. 2004, 47, 935–946. 10.1021/jm031016d. - DOI - PubMed
LinkOut - more resources
Full Text Sources
