Baicalin induces apoptosis and autophagy in human osteosarcoma cells by increasing ROS to inhibit PI3K/Akt/mTOR, ERK1/2 and β-catenin signaling pathways
- PMID: 35573641
- PMCID: PMC9091934
- DOI: 10.1016/j.jbo.2022.100415
Baicalin induces apoptosis and autophagy in human osteosarcoma cells by increasing ROS to inhibit PI3K/Akt/mTOR, ERK1/2 and β-catenin signaling pathways
Abstract
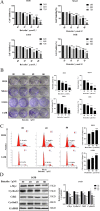
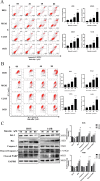
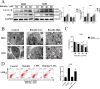
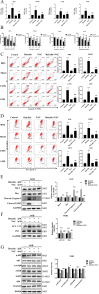
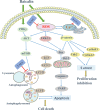
Baicalin, a flavonoid derivative, exerts antitumor activity in a variety of neoplasms. However, whether baicalin exerts antitumor effects on osteosarcoma cells remains to be elucidated. In this study, treatment with baicalin reduced the proliferation and invasive potential of osteosarcoma cells and reduced the mitochondrial membrane potential, which eventually caused mitochondrial apoptosis. In addition, baicalin increased intercellular Ca2+ and ROS concentrations. Baicalin-induced apoptosis was confirmed by enhanced Bax, cleaved caspase-3, and cleaved PARP levels and decreased Bcl-2 levels. The increase in LC3-II and p62 suggested that baicalin induced autophagosome formation but ultimately inhibited downstream autophagy. Moreover, apoptosis induced by baicalin was attenuated by the addition of 3-MA. Furthermore, we found that baicalin inhibited the PI3K/Akt/mTOR, ERK1/2 and β-catenin signaling pathways. Chelation of free Ca2+ by BAPTA-AM also inhibited both apoptosis induction and ROS concentration changes. Finally, NAC pretreatment reversed baicalin treatment outcomes, including the increase in Ca2+ concentration, induction of apoptosis and autophagy, and inhibition of the pathways. Molecular docking results indicated that baicalin might interact with the structural domain of PI3Kγ. Thus, baicalin may be considered a potential candidate for osteosarcoma treatment.
Keywords: Apoptosis; Autophagy; Baicalin; Osteosarcoma; PI3Kγ; ROS.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Raymond A., Jaffe N. Osteosarcoma multidisciplinary approach to the management from the pathologist's perspective. Cancer Treat. Res. 2009;152:63–84. - PubMed
-
- Gelberg K., Fitzgerald E., Hwang S., Dubrow R. Growth and development and other risk factors for osteosarcoma in children and young adults. Int. J. Epidemiol. 1997;26(2):272–278. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

