Identification of Synaptic DGKθ Interactors That Stimulate DGKθ Activity
- PMID: 35573662
- PMCID: PMC9095502
- DOI: 10.3389/fnsyn.2022.855673
Identification of Synaptic DGKθ Interactors That Stimulate DGKθ Activity
Abstract
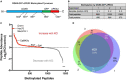
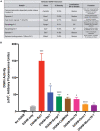
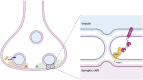
Lipids and their metabolic enzymes are a critical point of regulation for the membrane curvature required to induce membrane fusion during synaptic vesicle recycling. One such enzyme is diacylglycerol kinase θ (DGKθ), which produces phosphatidic acid (PtdOH) that generates negative membrane curvature. Synapses lacking DGKθ have significantly slower rates of endocytosis, implicating DGKθ as an endocytic regulator. Importantly, DGKθ kinase activity is required for this function. However, protein regulators of DGKθ's kinase activity in neurons have never been identified. In this study, we employed APEX2 proximity labeling and mass spectrometry to identify endogenous interactors of DGKθ in neurons and assayed their ability to modulate its kinase activity. Seven endogenous DGKθ interactors were identified and notably, synaptotagmin-1 (Syt1) increased DGKθ kinase activity 10-fold. This study is the first to validate endogenous DGKθ interactors at the mammalian synapse and suggests a coordinated role between DGKθ-produced PtdOH and Syt1 in synaptic vesicle recycling.
Keywords: APEX2; Syt1; lipids; neurotransmission; proximity labeling; synapse; synaptic vesicles.
Copyright © 2022 Barber, Goldschmidt, Ma, Devine, Cole, Huganir and Raben.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

