Refined Interpretation of the Pistillate Flower in Ceratophyllum Sheds Fresh Light on Gynoecium Evolution in Angiosperms
- PMID: 35573671
- PMCID: PMC9098228
- DOI: 10.3389/fcell.2022.868352
Refined Interpretation of the Pistillate Flower in Ceratophyllum Sheds Fresh Light on Gynoecium Evolution in Angiosperms
Abstract
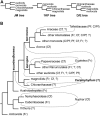
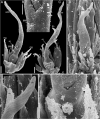
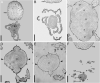
Molecular phylogenetic analyses have revealed a superclade of mesangiosperms with five extant lineages: monocots, eudicots, magnoliids, Ceratophyllum and Chloranthaceae. Both Ceratophyllum and Chloranthaceae are ancient lineages with a long fossil record; their precise placement within mesangiosperms is uncertain. Morphological studies have suggested that they form a clade together with some Cretaceous fossils, including Canrightia, Montsechia and Pseudoasterophyllites. Apart from Canrightia, members of this clade share unilocular gynoecia commonly interpreted as monomerous with ascidiate carpels. Alternatively, the gynoecium of Ceratophyllum has also been interpreted as syncarpous with a single fertile carpel (pseudomonomerous). We investigate patterns of morphological, anatomical and developmental variation in gynoecia of three Ceratophyllum species to explore the controversial interpretation of its gynoecium as either monomerous or pseudomonomerous. We use an angiosperm-wide morphological data set and contrasting tree topologies to estimate the ancestral gynoecium type in both Ceratophyllum and mesangiosperms. Gynoecia of all three Ceratophyllum species possess a small (sometimes vestigial) glandular appendage on the abaxial side and an occasionally bifurcating apex. The ovary is usually unilocular with two procambium strands, but sometimes bilocular and/or with three strands in C. demersum. None of the possible phylogenetic placements strongly suggest apocarpy in the stem lineage of Ceratophyllum. Rescoring Ceratophyllum as having two united carpels affects broader-scale reconstructions of the ancestral gynoecium in mesangiosperms. Our interpretation of the glandular appendage as a tepal or staminode homologue makes the Ceratophyllum ovary inferior, thus resembling (semi)inferior ovaries of most Chloranthaceae and potentially related fossils Canrightia and Zlatkocarpus. The entire structure of the flower of Ceratophyllum suggests strong reduction following a long and complex evolutionary history. The widely accepted notion that apocarpy is ancestral in mesangiosperms (and angiosperms) lacks robust support, regardless of which modes of carpel fusion are considered. Our study highlights the crucial importance of incorporating fossils into large-scale analyses to understand character evolution.
Keywords: Ceratophyllales; Chloranthales; apocarpy; congenital fusion; fossils; mesangiosperms; pseudomonomery; syncarpy.
Copyright © 2022 Sokoloff, El, Pechenyuk, Carrive, Nadot, Rudall and Remizowa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures











References
-
- Aboy H. E. (1936). A Study of the Anatomy and Morphology of Ceratophyllum demersum . MS Thesis. Ithaca: Cornell University.
-
- Antonov A. S., Troitsky A. V., Samigullin T. K., Bobrova V. K., Valiejo-Roman K. M., Martin W. (2000). “Early Events in the Evolution of Angiosperms Deduced from Cp rDNA ITS 2–4 Sequence Comparisons,” in Proceedings of the International Symposium on the Family Magnoliaceae. Editors Liu Y.-H., Fan H.-M., Chen Z.-Y., Wu Q.-G., Zeng Q.-W. (Beijing: Science Press; ), 210–214.
-
- Arber A. (1920). Water Plants: A Study of Aquatic Angiosperms. Cambridge: Cambridge University Press.
-
- Armbruster W. S., Debevec E. M., Willson M. F. (2002). Evolution of Syncarpy in Angiosperms: Theoretical and Phylogenetic Analyses of the Effects of Carpel Fusion on Offspring Quantity and Quality. J. Evol. Biol. 15, 657–672. 10.1046/j.1420-9101.2002.00414.x - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

