Phytochrome-Mediated Light Perception Affects Fruit Development and Ripening Through Epigenetic Mechanisms
- PMID: 35574124
- PMCID: PMC9096621
- DOI: 10.3389/fpls.2022.870974
Phytochrome-Mediated Light Perception Affects Fruit Development and Ripening Through Epigenetic Mechanisms
Abstract
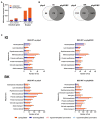
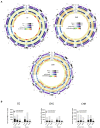
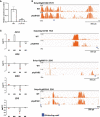
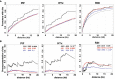
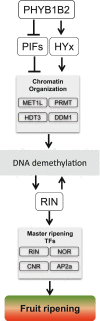
Phytochrome (PHY)-mediated light and temperature perception has been increasingly implicated as important regulator of fruit development, ripening, and nutritional quality. Fruit ripening is also critically regulated by chromatin remodeling via DNA demethylation, though the molecular basis connecting epigenetic modifications in fruits and environmental cues remains largely unknown. Here, to unravel whether the PHY-dependent regulation of fruit development involves epigenetic mechanisms, an integrative analysis of the methylome, transcriptome and sRNAome of tomato fruits from phyA single and phyB1B2 double mutants was performed in immature green (IG) and breaker (BK) stages. The transcriptome analysis showed that PHY-mediated light perception regulates more genes in BK than in the early stages of fruit development (IG) and that PHYB1B2 has a more substantial impact than PHYA in the fruit transcriptome, in both analyzed stages. The global profile of methylated cytosines revealed that both PHYA and PHYB1B2 affect the global methylome, but PHYB1B2 has a greater impact on ripening-associated methylation reprogramming across gene-rich genomic regions in tomato fruits. Remarkably, promoters of master ripening-associated transcription factors (TF) (RIN, NOR, CNR, and AP2a) and key carotenoid biosynthetic genes (PSY1, PDS, ZISO, and ZDS) remained highly methylated in phyB1B2 from the IG to BK stage. The positional distribution and enrichment of TF binding sites were analyzed over the promoter region of the phyB1B2 DEGs, exposing an overrepresentation of binding sites for RIN as well as the PHY-downstream effectors PIFs and HY5/HYH. Moreover, phyA and phyB1B2 mutants showed a positive correlation between the methylation level of sRNA cluster-targeted genome regions in gene bodies and mRNA levels. The experimental evidence indicates that PHYB1B2 signal transduction is mediated by a gene expression network involving chromatin organization factors (DNA methylases/demethylases, histone-modifying enzymes, and remodeling factors) and transcriptional regulators leading to altered mRNA profile of ripening-associated genes. This new level of understanding provides insights into the orchestration of epigenetic mechanisms in response to environmental cues affecting agronomical traits.
Keywords: DNA methylation; RdDM; carotenoid; chlorophyll; epigenetics; fleshy fruit; tomato.
Copyright © 2022 Bianchetti, Bellora, de Haro, Zuccarelli, Rosado, Freschi, Rossi and Bermudez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alves F. R. R., Lira B. S., Pikart F. C., Monteiro S. S., Furlan C. M., Purgatto E., et al. (2020). Beyond the limits of photoperception: constitutively active PHYTOCHROME B2 overexpression as a means of improving fruit nutritional quality in tomato. Plant Biotechnol. J. 18, 2027–2041. doi: 10.1111/pbi.13362, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

