Extracellular Matrix Protein 1 Regulates Colorectal Cancer Cell Proliferative, Migratory, Invasive and Epithelial-Mesenchymal Transition Activities Through the PI3K/AKT/GSK3β/Snail Signaling Axis
- PMID: 35574325
- PMCID: PMC9093678
- DOI: 10.3389/fonc.2022.889159
Extracellular Matrix Protein 1 Regulates Colorectal Cancer Cell Proliferative, Migratory, Invasive and Epithelial-Mesenchymal Transition Activities Through the PI3K/AKT/GSK3β/Snail Signaling Axis
Abstract
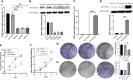
In prior reports, extracellular matrix protein 1 (ECM1) upregulation has been reported in colorectal cancer (CRC) patient tumor tissues, and has been suggested to be related to the metastatic progression of CRC, although the underlying mechanisms have yet to be clarified. In this study, we found that ECM1 was overexpressed in both CRC tissues and cell lines. Upregulation of ECM1 was correlated with tumor size, lymph node status and TNM stage in CRC patients. Knocking down ECM1 suppressed CRC cell growth, migration and invasion, in addition to reducing the expression of Vimentin and increasing E-cadherin expression. The overexpression of ECM1, in contrast, yielded the opposite phenotypic outcomes while also promoting the expression of p-AKT, p-GSK3β, and Snail, which were downregulated when ECM1 was knocked down. Treatment with LY294002 and 740 Y-P reversed the impact upregulation and downregulation of ECM1 on CRC cell metastasis and associated EMT induction. In vivo analyses confirmed that ECM1 overexpression was able to enhance EMT induction and CRC tumor progression. In conclusion, ECM1 influences CRC development and progression in an oncogenic manner, and regulates CRC metastasis and EMT processes via the PI3K/AKT/GSK3β/Snail signaling axis.
Keywords: Extracellular matrix protein 1; PI3K/AKT/GSK3β/Snail signaling pathway; colorectal cancer; epithelial-mesenchymal transition; metastasis.
Copyright © 2022 Long, Wang, Weng, Xiang and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

