The Role of RNA Methyltransferase METTL3 in Normal and Malignant Hematopoiesis
- PMID: 35574332
- PMCID: PMC9095908
- DOI: 10.3389/fonc.2022.873903
The Role of RNA Methyltransferase METTL3 in Normal and Malignant Hematopoiesis
Abstract
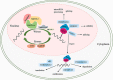
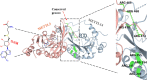
m6A modification is the most common modification in eukaryotes. METTL3, as a core methyltransferase of m6A modification, plays a vital role in normal and malignant hematopoiesis. Recent studies have shown that METTL3 is required for normal and symmetric differentiation of hematopoietic stem/progenitor cells (HSPCs). Moreover, METTL3 strongly impacts the process and development of hematological neoplasms, including the differentiation, apoptosis, proliferation, chemoresistance, and risk of tumors. Novel inhibitors of METTL3 have been identified and studied in acute myeloid leukemia (AML) cells. STM2457, a selective inhibitor of METTL3, has been identified to block proliferation and promote differentiation and apoptosis of AML cells without impacting normal hematopoiesis. Therefore, in our present review, we focus on the structure of METTL3, the role of METTL3 in both normal and malignant hematopoiesis, and the potential of METTL3 for treating hematological neoplasms.
Keywords: METTL3; N6-methyladenosine; inhibitor; malignant hematopoiesis; normal hematopoiesis.
Copyright © 2022 Wu, Ye and Gong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells.Nat Med. 2017 Nov;23(11):1369-1376. doi: 10.1038/nm.4416. Epub 2017 Sep 18. Nat Med. 2017. PMID: 28920958 Free PMC article.
-
The m6A RNA methyltransferase METTL3/METTL14 promotes leukemogenesis through the mdm2/p53 pathway in acute myeloid leukemia.J Cancer. 2022 Jan 4;13(3):1019-1030. doi: 10.7150/jca.60381. eCollection 2022. J Cancer. 2022. PMID: 35154467 Free PMC article.
-
Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia.Nature. 2021 May;593(7860):597-601. doi: 10.1038/s41586-021-03536-w. Epub 2021 Apr 26. Nature. 2021. PMID: 33902106 Free PMC article.
-
Novel insights into the m6A-RNA methyltransferase METTL3 in cancer.Biomark Res. 2021 Apr 20;9(1):27. doi: 10.1186/s40364-021-00278-9. Biomark Res. 2021. PMID: 33879256 Free PMC article. Review.
-
METTL3 plays multiple functions in biological processes.Am J Cancer Res. 2020 Jun 1;10(6):1631-1646. eCollection 2020. Am J Cancer Res. 2020. PMID: 32642280 Free PMC article. Review.
Cited by
-
METTL3 Affects Spinal Cord Neuronal Apoptosis by Regulating Bcl-2 m6A Modifications After Spinal Cord Injury.Neurospine. 2023 Jun;20(2):623-636. doi: 10.14245/ns.2346170.085. Epub 2023 Jun 30. Neurospine. 2023. PMID: 37401082 Free PMC article.
-
FUS and METTL3 collaborate to regulate RNA maturation, preventing unfolded protein response and promoting gastric cancer progression.Clin Exp Med. 2024 Dec 21;25(1):15. doi: 10.1007/s10238-024-01525-7. Clin Exp Med. 2024. PMID: 39708203 Free PMC article.
-
Dynamic Roles of RNA and RNA Epigenetics in HTLV-1 Biology.Viruses. 2025 Jan 17;17(1):124. doi: 10.3390/v17010124. Viruses. 2025. PMID: 39861913 Free PMC article. Review.
-
The impact of METTL3 on bladder cancer through m6A modification: a potential therapeutic target and prognostic biomarker.Front Oncol. 2025 Jul 3;15:1622117. doi: 10.3389/fonc.2025.1622117. eCollection 2025. Front Oncol. 2025. PMID: 40678060 Free PMC article. Review.
-
STM2457 Inhibits METTL3-Mediated m6A Modification of miR-30c to Alleviate Spinal Cord Injury by Inducing the ATG5-Mediated Autophagy.Neurospine. 2024 Sep;21(3):925-941. doi: 10.14245/ns.2448494.247. Epub 2024 Sep 30. Neurospine. 2024. PMID: 39363472 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources