Scalp-Sparing Radiation With Concurrent Temozolomide and Tumor Treating Fields (SPARE) for Patients With Newly Diagnosed Glioblastoma
- PMID: 35574391
- PMCID: PMC9106370
- DOI: 10.3389/fonc.2022.896246
Scalp-Sparing Radiation With Concurrent Temozolomide and Tumor Treating Fields (SPARE) for Patients With Newly Diagnosed Glioblastoma
Abstract
Introduction: Standard-of-care treatment for patients with newly diagnosed glioblastoma (GBM) after surgery or biopsy includes concurrent chemoradiation followed by maintenance temozolomide (TMZ) with tumor treating fields (TTFields). Preclinical studies suggest TTFields and radiotherapy work synergistically. We report the results of our trial evaluating the safety of TTFields used concurrently with chemoradiation.
Methods: This is a single-arm pilot study (clinicaltrials.gov Identifier: NCT03477110). Adult patients (age ≥ 18 years) with newly diagnosed glioblastoma and a Karnofsky performance score (KPS) of ≥ 60 were eligible. All patients received concurrent scalp-sparing radiation (60 Gy in 30 fractions) with TMZ (75 mg/m2 daily) and TTFields (200 kHz). Maintenance therapy included TMZ and continuation of TTFields. Scalp-sparing radiation treatment was used to reduce radiation dermatitis. Radiation treatment was delivered through the TTFields arrays. The primary endpoint was safety and toxicity of tri-modality treatment within 30 days of completion of chemoradiation treatment.
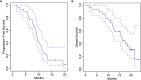
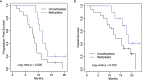
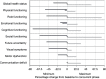
Results: There were 30 patients enrolled, including 20 (66.7%) men and 10 (33.3%) women, with a median age of 58 years (range 19 to 77 years). Median KPS was 90 (range 70 to 100). A total of 12 (40%) patients received a gross total resection and 18 (60%) patients had a subtotal resection. A total of 12 (40%) patients had multifocal disease at presentation. There were 20 (66.7%) patients who had unmethylated O(6)-methylguanine-DNA-methyltransferase (MGMT) promotor status and 10 (33.3%) patients who had methylated MGMT promoter status. Median follow-up was 15.2 months (range 1.7 to 23.6 months). Skin adverse events were noted in 83.3% of patients, however, these were limited to Grade 1 or 2 events, which resolved spontaneously or with topical medications. The primary end point was met; no TTFields discontinuation occurred during the evaluation period due to high grade scalp toxicity. A total of 27 (90%) patients had progression, with a median progression-free survival (PFS) of 9.3 months (95% confidence interval (CI): 8.5-11.6 months). The 1-year progression-free survival was 23% (95% CI: 12%-45%). The median overall survival (OS) was 15.8 months (95% CI: 12.5 months-infinity). The 1-year overall survival was 66% (95% CI: 51%-86%).
Conclusions: Concurrent TTFields with scalp-sparing chemoradiation is a feasible and well-tolerated treatment option with limited toxicity. A phase 3, randomized clinical trial (EF-32, clinicaltrials.gov Identifier: NCT04471844) investigating the clinical benefit of concurrent TTFields with chemoradiation treatment is currently enrolling.
Clinical trial registration: Clinicaltrials.gov, identifier NCT03477110.
Keywords: TTFields; concurrent therapy; glioblastoma; radiotherapy; scalp-sparing radiation.
Copyright © 2022 Miller, Song, Ali, Niazi, Bar-Ad, Martinez, Glass, Alnahhas, Andrews, Judy, Evans, Farrell, Werner-Wasik, Chervoneva, Ly, Palmer, Liu and Shi.
Conflict of interest statement
WS: Consulting for Brainlab, Varian, and Novocure; research funding for clinical trial from Novocure and Regeneron. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Concurrent chemoradiation and Tumor Treating Fields (TTFields, 200 kHz) for patients with newly diagnosed glioblastoma: patterns of progression in a single institution pilot study.J Neurooncol. 2022 Nov;160(2):345-350. doi: 10.1007/s11060-022-04146-w. Epub 2022 Nov 10. J Neurooncol. 2022. PMID: 36355259 Clinical Trial.
-
Initial experience with scalp sparing radiation with concurrent temozolomide and tumor treatment fields (SPARE) for patients with newly diagnosed glioblastoma.J Neurooncol. 2020 May;147(3):653-661. doi: 10.1007/s11060-020-03466-z. Epub 2020 Mar 23. J Neurooncol. 2020. PMID: 32206976 Clinical Trial.
-
Tumor treating fields with radiation for glioblastoma: a narrative review.Chin Clin Oncol. 2022 Oct;11(5):40. doi: 10.21037/cco-22-90. Chin Clin Oncol. 2022. PMID: 36336899 Review.
-
Concurrent Tumor Treating Fields (TTFields) and Radiation Therapy for Newly Diagnosed Glioblastoma: A Prospective Safety and Feasibility Study.Front Oncol. 2020 Apr 21;10:411. doi: 10.3389/fonc.2020.00411. eCollection 2020. Front Oncol. 2020. PMID: 32373508 Free PMC article.
-
The Evolving Role of Tumor Treating Fields in Managing Glioblastoma: Guide for Oncologists.Am J Clin Oncol. 2018 Feb;41(2):191-196. doi: 10.1097/COC.0000000000000395. Am J Clin Oncol. 2018. PMID: 28832384 Free PMC article. Review.
Cited by
-
Phase 1 study of concomitant tumor treating fields and temozolomide chemoradiation for newly diagnosed glioblastoma.Neurooncol Adv. 2024 Jul 29;6(1):vdae129. doi: 10.1093/noajnl/vdae129. eCollection 2024 Jan-Dec. Neurooncol Adv. 2024. PMID: 39211521 Free PMC article.
-
Therapeutic potential of tumor treating fields for malignant brain tumors.Cancer Rep (Hoboken). 2023 May;6(5):e1813. doi: 10.1002/cnr2.1813. Epub 2023 Mar 29. Cancer Rep (Hoboken). 2023. PMID: 36987739 Free PMC article. Review.
-
Concurrent Tumor-Treating Fields and Chemoradiotherapy: Outcomes in Grade 4 Glioma Patients.Clin Med Insights Oncol. 2025 Feb 24;19:11795549251315579. doi: 10.1177/11795549251315579. eCollection 2025. Clin Med Insights Oncol. 2025. PMID: 40007556 Free PMC article.
-
Meta-Analysis of Modulated Electro-Hyperthermia and Tumor Treating Fields in the Treatment of Glioblastomas.Cancers (Basel). 2023 Jan 31;15(3):880. doi: 10.3390/cancers15030880. Cancers (Basel). 2023. PMID: 36765840 Free PMC article. Review.
-
Concurrent chemoradiation and Tumor Treating Fields (TTFields, 200 kHz) for patients with newly diagnosed glioblastoma: patterns of progression in a single institution pilot study.J Neurooncol. 2022 Nov;160(2):345-350. doi: 10.1007/s11060-022-04146-w. Epub 2022 Nov 10. J Neurooncol. 2022. PMID: 36355259 Clinical Trial.
References
-
- National Comprehensive Cancer Network . Central Nervous System Cancers (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (Accessed March 1, 2022).
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. . Effects of Radiotherapy With Concomitant and Adjuvant Temozolomide Versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol (2009) 10:459–66. doi: 10.1016/S1470-2045(09)70025-7 - DOI - PubMed
-
- Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, et al. . Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA (2017) 318:2306–16. doi: 10.1001/jama.2017.18718 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

