A Single-Arm Phase 2 Trial on Induction Chemotherapy Followed by Concurrent Chemoradiation in Nasopharyngeal Carcinoma Using a Reduced Cumulative Dose of Cisplatin
- PMID: 35574402
- PMCID: PMC9092977
- DOI: 10.3389/fonc.2022.842281
A Single-Arm Phase 2 Trial on Induction Chemotherapy Followed by Concurrent Chemoradiation in Nasopharyngeal Carcinoma Using a Reduced Cumulative Dose of Cisplatin
Abstract
Background: We conducted this study to evaluate if a reduced cumulative dose of induction and concurrent cisplatin conferred similar favorable outcomes when compared to trial NPC-0501.
Methods: Newly diagnosed nasopharyngeal carcinoma (NPC) with stage III-IVA were prospectively recruited from January 2015 to September 2019. Induction chemotherapy (IC) consisted of cisplatin 80mg/m2 on day 1 and capecitabine 1000mg/m2 twice daily from day 1 to 14 every 3 weeks for 3 cycles followed by concurrent chemoradiotherapy (CCRT) with 2 cycles of cisplatin 100mg/m2 given every 3 weeks. Tumor response was evaluated according to RECIST v1.1. Acute and late adverse events (AEs) were graded with CTCAE v4.0 and Late Radiation Morbidity Scoring of the RTOG, respectively.
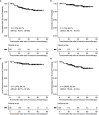
Results: 135 patients were recruited. At 16 weeks after CCRT, all 130 patients who completed the entire course of radiotherapy (RT) had a complete response upon final assessment. With a median follow-up of 36.2 months, 22 treatment failures and 8 deaths were observed. The 3-year progression-free survival, overall survival, locoregional recurrence-free survival, and distant recurrence-free survival were 83.7%, 94.1%, 94.1%, and 85.9%, respectively. Our survival data outcomes were similar to those reported in the cisplatin and capecitabine (PX) induction arm of the 0501 trial. 103 patients (76.3%) reported acute grade 3-4 AEs. Two patients (1.5%) had late grade 3-4 complications, numerically fewer than those reported in the NPC-0501 trial.
Conclusions: Induction PX and concurrent cisplatin with a reduced cumulative cisplatin dose yield survival outcomes comparable to those reported in the NPC-0501 trial with excellent tolerability. Therefore, a reduced cumulative dose of cisplatin is a promising treatment scheme for nasopharyngeal carcinoma.
Keywords: capecitabine; cisplatin; induction chemotherapy; nasopharyngeal carcinoma; progression-free survival.
Copyright © 2022 Xu, Yang, Ng, Helali, Lee, Ma, Liu, Li, Shen, Huang, Zha, Zhou, Lee and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- International Agency for Research on Cancer (IARC) . Global Cancer Observatory (2020). World Health Organization. Available at: https://gco.iarc.fr/ (Accessed September 20, 2020).
LinkOut - more resources
Full Text Sources