Fcγ-Receptor-Based Enzyme-Linked Immunosorbent Assays for Sensitive, Specific, and Persistent Detection of Anti-SARS-CoV-2 Nucleocapsid Protein IgG Antibodies in Human Sera
- PMID: 35574677
- PMCID: PMC9199419
- DOI: 10.1128/jcm.00075-22
Fcγ-Receptor-Based Enzyme-Linked Immunosorbent Assays for Sensitive, Specific, and Persistent Detection of Anti-SARS-CoV-2 Nucleocapsid Protein IgG Antibodies in Human Sera
Abstract
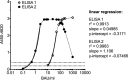
Sensitive and specific serological tests are mandatory for epidemiological studies evaluating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) prevalence as well as coronavirus disease 2019 (COVID-19) morbidity and mortality rates. The accuracy of results is challenged by antibody waning after convalescence and by cross-reactivity induced by previous infections with other pathogens. By employing a patented platform technology based on capturing antigen-antibody complexes with a solid-phase-bound Fcγ receptor (FcγR) and truncated nucleocapsid protein as the antigen, two SARS-CoV-2 IgG enzyme-linked immunosorbent assays (ELISAs), featuring different serum and antigen dilutions, were developed. Validation was performed using a serum panel comprising 213 longitudinal samples from 35 COVID-19 patients and a negative-control panel consisting of 790 pre-COVID-19 samples from different regions of the world. While both assays show similar diagnostic sensitivities in the early convalescent phase, ELISA 2 (featuring a higher serum concentration) enables SARS-CoV-2 IgG antibody detection for a significantly longer time postinfection (≥15 months). Correspondingly, analytical sensitivity referenced to indirect immunofluorescence testing (IIFT) is significantly higher for ELISA 2 in samples with a titer of ≤1:640; for high-titer samples, a prozone effect is observed for ELISA 2. The specificities of both ELISAs were excellent not only for pre-COVID-19 serum samples from Europe, Asia, and South America but also for several challenging African sample panels. The SARS-CoV-2 IgG FcγR ELISAs, methodically combining antigen-antibody binding in solution and isotype-specific detection of immune complexes, are valuable tools for seroprevalence studies requiring the (long-term) detection of anti-SARS-CoV-2 IgG antibodies in populations with a challenging immunological background and/or in which spike-protein-based vaccine programs have been rolled out.
Keywords: immunoassay; immunoglobulins; infectious disease; laboratory methods and tools; viral diseases.
Conflict of interest statement
The authors declare a conflict of interest. Research Funding: C. Deschermeier, German Federal Ministry of Education and Research (grant no. 01KI20210) and European Regional Development Fund (ERDF) (grant no. BWF/H/52228/2012/13.10.10-1/3.4,6) to institution; P. Emmerich, German Federal Ministry of Education and Research (grant no. 01KI20210) to institution; L. Oestereich: Leibniz Association (grant no. J59/20218) to institution. Patents: C. Deschermeier, EP3207375; P. Emmerich, EP2492689, EP3207375. Other financial or non-financial interests: C. Deschermeier and B. Rushton are cofounders and shareholders of Panadea Diagnostics GmbH. Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript. No other Potential Conflicts of Interest have been declared.
Figures



References
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, Adriano A, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Dittrich S, Emperador D, Hooft L, Leeflang MMG, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group . 2020. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 6:CD013652. doi:10.1002/14651858.CD013652. - DOI - PMC - PubMed
-
- Emmerich P, Murawski C, Ehmen C, von Possel R, Pekarek N, Oestereich L, Duraffour S, Pahlmann M, Struck N, Eibach D, Krumkamp R, Amuasi J, Maiga‐Ascofaré O, Rakotozandrindrainy R, Asogun D, Ighodalo Y, Kann S, May J, Tannich E, Deschermeier C. 2021. Limited specificity of commercially available SARS-CoV-2 IgG ELISAs in serum samples of African origin. Trop Med Int Health 26:621–631. doi:10.1111/tmi.13569. - DOI - PMC - PubMed
-
- Nkuba Ndaye A, Hoxha A, Madinga J, Marien J, Peeters M, Leendertz FH, Ahuka Mundeke S, Arien KK, Muyembe Tanfumu J-J, Mbala Kingebeni P, Vanlerberghe V. 2021. Challenges in interpreting SARS-CoV-2 serological results in African countries. Lancet Glob Health 9:e588–e589. doi:10.1016/S2214-109X(21)00060-7. - DOI - PMC - PubMed
-
- Yadouleton A, Sander A-L, Moreira-Soto A, Tchibozo C, Hounkanrin G, Badou Y, Fischer C, Krause N, Akogbeto P, de Oliveira Filho EF, Dossou A, Brunink S, Aissi MAJ, Djingarey MH, Hounkpatin B, Nagel M, Drexler JF. 2021. Limited specificity of serologic tests for SARS-CoV-2 antibody detection, Benin. Emerg Infect Dis 27:233–237. doi:10.3201/eid2701.203281. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

