A first-in-human Phase I trial of the oral p-STAT3 inhibitor WP1066 in patients with recurrent malignant glioma
- PMID: 35575067
- PMCID: PMC9134932
- DOI: 10.2217/cns-2022-0005
A first-in-human Phase I trial of the oral p-STAT3 inhibitor WP1066 in patients with recurrent malignant glioma
Abstract
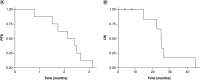
Aim: To ascertain the maximum tolerated dose (MTD)/maximum feasible dose (MFD) of WP1066 and p-STAT3 target engagement within recurrent glioblastoma (GBM) patients. Patients & methods: In a first-in-human open-label, single-center, single-arm 3 + 3 design Phase I clinical trial, eight patients were treated with WP1066 until disease progression or unacceptable toxicities. Results: In the absence of significant toxicity, the MFD was identified to be 8 mg/kg. The most common adverse event was grade 1 nausea and diarrhea in 50% of patients. No treatment-related deaths occurred; 6 of 8 patients died from disease progression and one was lost to follow-up. Of 8 patients with radiographic follow-up, all had progressive disease. The longest response duration exceeded 3.25 months. The median progression-free survival (PFS) time was 2.3 months (95% CI: 1.7 months-NA months), and 6-month PFS (PFS6) rate was 0%. The median overall survival (OS) rate was 25 months (95% CI: 22.5 months-NA months), with an estimated 1-year OS rate of 100%. Pharmacokinetic (PK) data demonstrated that at 8 mg/kg, the T1/2 was 2-3 h with a dose dependent increase in the Cmax. Immune monitoring of the peripheral blood demonstrated that there was p-STAT3 suppression starting at a dose of 1 mg/kg. Conclusion: Immune analyses indicated that WP1066 inhibited systemic immune p-STAT3. WP1066 had an MFD identified at 8 mg/kg which is the target allometric dose based on prior preclinical modeling in combination with radiation therapy and a Phase II study is being planned for newly diagnosed MGMT promoter unmethylated glioblastoma patients.
Trial registration: ClinicalTrials.gov NCT02977780.
Keywords: Phase I; STAT3 inhibitor; glioblastoma; toxicity.
Conflict of interest statement
Financial & competing interests disclosure
Support for this research was received from The Ben and Catherine Ivy Foundation and The University of Texas MD Anderson Moon Shot Program. Amy B Heimberger has licensed intellectual property to Celldex Therapeutics and DNAtrix. She serves on the advisory board of Caris Life Sciences, WCG Oncology, owns stock in Caris Life Sciences and has received research funding from Celularity Inc. and Codiak BioScience Inc. Moleculin has licensed WP1066 from MD Anderson Cancer Center that was created by Waldemar Priebe and provided study drug and reimbursement for PK analysis. Study supported by NIH grants: CA120813, NS120547, CA093459 and Cancer Prevention Research Institute of Texas (CPRIT) IIRA RP160482. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352(10), 987–996 (2005). - PubMed
-
- Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin. Cancer Res. 13(5), 1362–1366 (2007). - PubMed
-
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat. Rev. Cancer 4(2), 97–105 (2004). - PubMed
-
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 7(1), 41–51 (2007). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
