Gastrointestinal neoplasia: carcinogenic interaction between bile acids and Helicobacter pylori in the stomach
- PMID: 35575088
- PMCID: PMC9106340
- DOI: 10.1172/JCI160194
Gastrointestinal neoplasia: carcinogenic interaction between bile acids and Helicobacter pylori in the stomach
Abstract
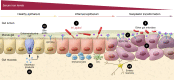
Bile acids modulate cell functions in health and disease, however, the mechanisms underlying their actions on neoplastic cells in the gastrointestinal (GI) tract remain largely unknown. In this issue of the JCI, Noto et al. comprehensively analyzed how interactions between Helicobacter pylori infection, iron deficiency, and bile acids modulate gastric inflammation and carcinogenesis. The investigators used sophisticated models, including INS-GAS mice with elevated serum gastrin and gastric acid secretion, in which H. pylori infection mimics human disease progression, to show that selected bile acids potentiated the carcinogenic effects of H. pylori infection and iron depletion. This elegant work has broad translational implications for microbe-associated GI neoplasia. Importantly, bile acid sequestration robustly attenuated the combined effects of H. pylori infection and iron depletion on gastric inflammation and cancer.
Conflict of interest statement
Figures
Comment on
-
Iron deficiency linked to altered bile acid metabolism promotes Helicobacter pylori-induced inflammation-driven gastric carcinogenesis.J Clin Invest. 2022 May 16;132(10):e147822. doi: 10.1172/JCI147822. J Clin Invest. 2022. PMID: 35316215 Free PMC article.

