Limited Dose-Dependent Effects of Vedolizumab on Various Leukocyte Subsets
- PMID: 35575178
- PMCID: PMC9236604
- DOI: 10.14309/ctg.0000000000000494
Limited Dose-Dependent Effects of Vedolizumab on Various Leukocyte Subsets
Abstract
Objectives: The anti-α4β7 integrin antibody vedolizumab (VDZ) is successfully used for the treatment of inflammatory bowel diseases. However, only a subgroup of patients respond to therapy. VDZ is administered at a fixed dose, leading to a wide range of serum concentrations in patients. Previous work from our group showed a dose-dependent preferential binding of VDZ to effector compared with regulatory CD4 + T cells. Therefore, we aimed to determine the dose-dependent binding profile of VDZ to other leukocyte subsets.
Methods: We characterized α4β7 integrin expression on CD8 + T cells, CD19 + B cells, CD14 + monocytes, natural killer cells, and eosinophils from patients with inflammatory bowel disease and healthy controls. We studied the binding of VDZ to these cells at different concentrations and investigated the functional consequences for dynamic adhesion and transmigration in vitro .
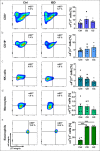
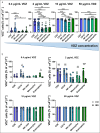
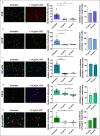
Results: The expression of α4β7 differed between the analyzed leukocyte subsets and was significantly higher on eosinophils from inflammatory bowel disease patients compared with controls. Almost all α4β7-expressing cells from these subsets were bound by VDZ at a concentration of 10 μg/mL. Dynamic cell adhesion was significantly impaired in all subsets, but there were no dose-dependent differences in the inhibition of adhesion.
Discussion: Our data suggest that α4β7-expressing CD8 + T cells, CD19 + B cells, CD14 + monocytes, natural killer cells, and eosinophils are a target of VDZ. However, there do not seem to be concentration-dependent differences, regarding the effects on these cells in the clinically relevant range. Thus, the reported exposure-efficacy characteristic of VDZ can probably mainly be attributed to CD4 + T-cell subsets.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- Zundler S, Becker E, Lou SchulzeL, et al. . Immune cell trafficking and retention in inflammatory bowel disease: Mechanistic insights and therapeutic advances. Gut 2019;68(9):1688–700. doi: - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, et al. . Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7(9):678–89. doi: - PubMed
-
- Feagan BG, Rutgeerts P, Sands BE, et al. . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369(8):699–710. doi: - PubMed
-
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. . Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369(8):711–21. doi: - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

