Circadian clocks of the kidney: function, mechanism, and regulation
- PMID: 35575250
- PMCID: PMC9273266
- DOI: 10.1152/physrev.00045.2021
Circadian clocks of the kidney: function, mechanism, and regulation
Abstract
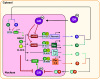
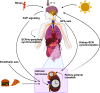
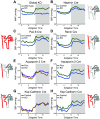
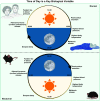
An intrinsic cellular circadian clock is located in nearly every cell of the body. The peripheral circadian clocks within the cells of the kidney contribute to the regulation of a variety of renal processes. In this review, we summarize what is currently known regarding the function, mechanism, and regulation of kidney clocks. Additionally, the effect of extrarenal physiological processes, such as endocrine and neuronal signals, on kidney function is also reviewed. Circadian rhythms in renal function are an integral part of kidney physiology, underscoring the importance of considering time of day as a key biological variable. The field of circadian renal physiology is of tremendous relevance, but with limited physiological and mechanistic information on the kidney clocks this is an area in need of extensive investigation.
Keywords: chronic kidney disease; renal; rhythm.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


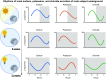
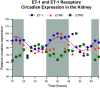
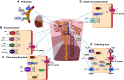
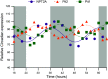
Similar articles
-
Circadian rhythms and the kidney.Nat Rev Nephrol. 2018 Oct;14(10):626-635. doi: 10.1038/s41581-018-0048-9. Nat Rev Nephrol. 2018. PMID: 30143787 Review.
-
The Circadian Clock in the Regulation of Renal Rhythms.J Biol Rhythms. 2015 Dec;30(6):470-86. doi: 10.1177/0748730415610879. Epub 2015 Nov 2. J Biol Rhythms. 2015. PMID: 26527094 Free PMC article. Review.
-
Endocrine regulation of circadian physiology.J Endocrinol. 2016 Jul;230(1):R1-R11. doi: 10.1530/JOE-16-0051. Epub 2016 Apr 22. J Endocrinol. 2016. PMID: 27106109 Review.
-
Interactions between endocrine and circadian systems.J Mol Endocrinol. 2013 Dec 19;52(1):R1-16. doi: 10.1530/JME-13-0118. Print 2014 Feb. J Mol Endocrinol. 2013. PMID: 23997239 Review.
-
Circadian regulation of renal function.Free Radic Biol Med. 2018 May 1;119:93-107. doi: 10.1016/j.freeradbiomed.2018.01.018. Epub 2018 Jan 31. Free Radic Biol Med. 2018. PMID: 29360554 Free PMC article. Review.
Cited by
-
Circadian gene BMAL1 ameliorates renal ischaemia-reperfusion injury in diabetic mice by enhancing mitophagy via the HIF-1/BNIP3 pathway.Sci Rep. 2025 Jul 2;15(1):23001. doi: 10.1038/s41598-025-03515-5. Sci Rep. 2025. PMID: 40594206 Free PMC article.
-
Toward Precision Medicine: Circadian Rhythm of Blood Pressure and Chronotherapy for Hypertension - 2021 NHLBI Workshop Report.Hypertension. 2023 Mar;80(3):503-522. doi: 10.1161/HYPERTENSIONAHA.122.19372. Epub 2022 Nov 30. Hypertension. 2023. PMID: 36448463 Free PMC article. Review.
-
Recent advances in understanding the kidney circadian clock mechanism.Am J Physiol Renal Physiol. 2024 Mar 1;326(3):F382-F393. doi: 10.1152/ajprenal.00214.2023. Epub 2024 Jan 4. Am J Physiol Renal Physiol. 2024. PMID: 38174377 Free PMC article. Review.
-
Impact of Long-Term Cannabidiol (CBD) Treatment on Mouse Kidney Transcriptome.Genes (Basel). 2024 Dec 21;15(12):1640. doi: 10.3390/genes15121640. Genes (Basel). 2024. PMID: 39766907 Free PMC article.
-
Circadian gene expression in mouse renal proximal tubule.Am J Physiol Renal Physiol. 2023 Mar 1;324(3):F301-F314. doi: 10.1152/ajprenal.00231.2022. Epub 2023 Feb 2. Am J Physiol Renal Physiol. 2023. PMID: 36727945 Free PMC article.
References
-
- Cederroth CR, Albrecht U, Bass J, Brown SA, Dyhrfjeld-Johnsen J, Gachon F, Green CB, Hastings MH, Helfrich-Förster C, Hogenesch JB, Lévi F, Loudon A, Lundkvist GB, Meijer JH, Rosbash M, Takahashi JS, Young M, Canlon B. Medicine in the fourth dimension. Cell Metab 30: 238–250, 2019. doi:10.1016/j.cmet.2019.06.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

