Functions of the FGF signalling pathway in cephalochordates provide insight into the evolution of the prechordal plate
- PMID: 35575387
- PMCID: PMC9188755
- DOI: 10.1242/dev.200252
Functions of the FGF signalling pathway in cephalochordates provide insight into the evolution of the prechordal plate
Abstract
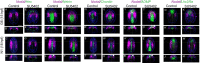
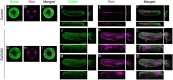
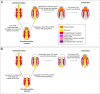
The fibroblast growth factor (FGF) signalling pathway plays various roles during vertebrate embryogenesis, from mesoderm formation to brain patterning. This diversity of functions relies on the fact that vertebrates possess the largest FGF gene complement among metazoans. In the cephalochordate amphioxus, which belongs to the chordate clade together with vertebrates and tunicates, we have previously shown that the main role of FGF during early development is the control of rostral somite formation. Inhibition of this signalling pathway induces the loss of these structures, resulting in an embryo without anterior segmented mesoderm, as in the vertebrate head. Here, by combining several approaches, we show that the anterior presumptive paraxial mesoderm cells acquire an anterior axial fate when FGF signal is inhibited and that they are later incorporated in the anterior notochord. Our analysis of notochord formation in wild type and in embryos in which FGF signalling is inhibited also reveals that amphioxus anterior notochord presents transient prechordal plate features. Altogether, our results give insight into how changes in FGF functions during chordate evolution might have participated to the emergence of the complex vertebrate head.
Keywords: Amphioxus; Brachyury; Goosecoid; Head mesoderm; Notochord.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Similar articles
-
Genetic regulation of amphioxus somitogenesis informs the evolution of the vertebrate head mesoderm.Nat Ecol Evol. 2019 Aug;3(8):1233-1240. doi: 10.1038/s41559-019-0933-z. Epub 2019 Jul 1. Nat Ecol Evol. 2019. PMID: 31263232
-
Amphioxus FGF signaling predicts the acquisition of vertebrate morphological traits.Proc Natl Acad Sci U S A. 2011 May 31;108(22):9160-5. doi: 10.1073/pnas.1014235108. Epub 2011 May 12. Proc Natl Acad Sci U S A. 2011. PMID: 21571634 Free PMC article.
-
Amphioxus goosecoid and the evolution of the head organizer and prechordal plate.Evol Dev. 2000 Nov-Dec;2(6):303-10. doi: 10.1046/j.1525-142x.2000.00073.x. Evol Dev. 2000. PMID: 11256375
-
Is There a Prechordal Region and an Acroterminal Domain in Amphioxus?Brain Behav Evol. 2022;96(4-6):334-352. doi: 10.1159/000521966. Epub 2022 Jan 14. Brain Behav Evol. 2022. PMID: 35034027 Review.
-
Metamerism in cephalochordates and the problem of the vertebrate head.Int J Dev Biol. 2017;61(10-11-12):621-632. doi: 10.1387/ijdb.170121to. Int J Dev Biol. 2017. PMID: 29319111 Review.
Cited by
-
Exploring tissue morphodynamics using the photoconvertible Kaede protein in amphioxus embryos.PLoS One. 2022 Sep 27;17(9):e0275193. doi: 10.1371/journal.pone.0275193. eCollection 2022. PLoS One. 2022. PMID: 36166455 Free PMC article.
-
Cell type and regulatory analysis in amphioxus illuminates evolutionary origin of the vertebrate head.Nat Commun. 2024 Oct 14;15(1):8859. doi: 10.1038/s41467-024-52938-7. Nat Commun. 2024. PMID: 39402029 Free PMC article.
-
An amphioxus neurula stage cell atlas supports a complex scenario for the emergence of vertebrate head mesoderm.Nat Commun. 2024 May 29;15(1):4550. doi: 10.1038/s41467-024-48774-4. Nat Commun. 2024. PMID: 38811547 Free PMC article.
-
Deep homology of a brachyury cis-regulatory syntax and the evolutionary origin of the notochord.Sci Adv. 2025 Jul 25;11(30):eadw3307. doi: 10.1126/sciadv.adw3307. Epub 2025 Jul 25. Sci Adv. 2025. PMID: 40712008 Free PMC article.
-
Amphioxus as a model to study the evolution of development in chordates.Elife. 2023 Sep 18;12:e87028. doi: 10.7554/eLife.87028. Elife. 2023. PMID: 37721204 Free PMC article.
References
-
- Albuixech-Crespo, B., López-Blanch, L., Burguera, D., Maeso, I., Sánchez-Arrones, L., Moreno-Bravo, J. A., Somorjai, I., Pascual-Anaya, J., Puelles, E., Bovolenta, P.et al. (2017). Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLoS Biol. 15, e2001573. 10.1371/journal.pbio.2001573 - DOI - PMC - PubMed
-
- Aldea, D., Subirana, L., Keime, C., Meister, L., Maeso, I., Marcellini, S., Gomez-Skarmeta, J. L., Bertrand, S. and Escriva, H. (2019). Genetic regulation of amphioxus somitogenesis informs the evolution of the vertebrate head mesoderm. Nat. Ecol. Evol. 3, 1233-1240. 10.1038/s41559-019-0933-z - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

