Red ginseng polysaccharide exhibits anticancer activity through GPX4 downregulation-induced ferroptosis
- PMID: 35575436
- PMCID: PMC9116236
- DOI: 10.1080/13880209.2022.2066139
Red ginseng polysaccharide exhibits anticancer activity through GPX4 downregulation-induced ferroptosis
Abstract
Context: Red ginseng polysaccharide (RGP) is an active component of the widely used medicinal plant Panax ginseng C. A. Meyer (Araliaceae), which has displayed promising activities against cancer cells. However, the detailed molecular mechanism of RGP in ferroptosis is still unknown.
Objective: This study evaluates the effects of RGP in cancer cells.
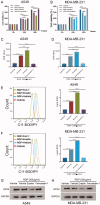
Materials and methods: A549 and MDA-MB-231 cells were used. Cell proliferation was measured by CCK-8 assay after being treated with RGP at concentrations of 0, 50, 100, 200, 400, 800 and 1600 μg/mL at 0, 12, 24 and 48 h. Lipid reactive oxygen species (ROS) levels were assessed by C11-BODIPY assay. The control group was treated with PBS.
Results: RGP inhibited human A549 (IC50: 376.2 μg/mL) or MDA-MB-231(IC50: 311.3 μg/mL) proliferation and induced lactate dehydrogenase (LDH) release, promoted ferroptosis and suppressed the expression of GPX4. Moreover, the effects of RGP were enhanced by the ferroptosis inducer erastin, while abolished by ferroptosis inhibitor ferrostatin-1.
Discussion and conclusions: Our study is the first to demonstrate (1) the anticancer activity of RGP in human lung cancer and breast cancer. (2) RGP presented the anti-ferroptosis effects in lung and breast cancer cells via targeting GPX4.
Keywords: Lung cancer; breast cancer; traditional Chinese medicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Corbit R, Ebbs S, King ML, Murphy LL.. 2006. The influence of lead and arsenite on the inhibition of human breast cancer MCF-7 cell proliferation by American ginseng root (Panax quinquefolius L.). Life Sci. 78(12):1336–1340. - PubMed
-
- Dixon SJ. 2017. Ferroptosis: bug or feature? Immunol Rev. 277(1):150–157. - PubMed
-
- Dixon SJ, Stockwell BR.. 2019. The hallmarks of ferroptosis. Annu Rev Cancer Biol. 3(1):35–54.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
