Adenosine, caffeine, and sleep-wake regulation: state of the science and perspectives
- PMID: 35575450
- PMCID: PMC9541543
- DOI: 10.1111/jsr.13597
Adenosine, caffeine, and sleep-wake regulation: state of the science and perspectives
Abstract
For hundreds of years, mankind has been influencing its sleep and waking state through the adenosinergic system. For ~100 years now, systematic research has been performed, first started by testing the effects of different dosages of caffeine on sleep and waking behaviour. About 70 years ago, adenosine itself entered the picture as a possible ligand of the receptors where caffeine hooks on as an antagonist to reduce sleepiness. Since the scientific demonstration that this is indeed the case, progress has been fast. Today, adenosine is widely accepted as an endogenous sleep-regulatory substance. In this review, we discuss the current state of the science in model organisms and humans on the working mechanisms of adenosine and caffeine on sleep. We critically investigate the evidence for a direct involvement in sleep homeostatic mechanisms and whether the effects of caffeine on sleep differ between acute intake and chronic consumption. In addition, we review the more recent evidence that adenosine levels may also influence the functioning of the circadian clock and address the question of whether sleep homeostasis and the circadian clock may interact through adenosinergic signalling. In the final section, we discuss the perspectives of possible clinical applications of the accumulated knowledge over the last century that may improve sleep-related disorders. We conclude our review by highlighting some open questions that need to be answered, to better understand how adenosine and caffeine exactly regulate and influence sleep.
Keywords: chronic caffeine; circadian; genetics; sleep deprivation; sleep homeostasis; sleep-wake disorder.
© 2022 The Authors. Journal of Sleep Research published by John Wiley & Sons Ltd on behalf of European Sleep Research Society.
Conflict of interest statement
All authors declare not having any conflicts of interest.
Figures

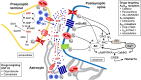

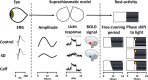
References
-
- Argyropoulos, I. T. , Samakouri, M. A. , Balascas, D. K. , Dalapascha, M. , Pallas, D. P. , & Livaditis, M. D. (2005). Mental health problems of Army personnel seen in medical outpatient clinics in Greece. International Journal of Psychiatry in Medicine, 35(3), 225–239. 10.2190/B6XN-W4CT-YPV0-6JFE - DOI - PubMed
-
- Atack, J. R. , Shook, B. C. , Rassnick, S. , Jackson, P. F. , Rhodes, K. , Drinkenburg, W. H. , … Megens, A. A. (2014). JNJ‐40255293, a novel adenosine A2A/A1 antagonist with efficacy in preclinical models of Parkinson's disease. ACS Chemical Neuroscience, 5(10), 1005–1019. 10.1021/cn5001606 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

