Stereospecific lasofoxifene derivatives reveal the interplay between estrogen receptor alpha stability and antagonistic activity in ESR1 mutant breast cancer cells
- PMID: 35575456
- PMCID: PMC9177151
- DOI: 10.7554/eLife.72512
Stereospecific lasofoxifene derivatives reveal the interplay between estrogen receptor alpha stability and antagonistic activity in ESR1 mutant breast cancer cells
Abstract
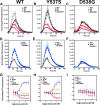
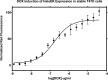
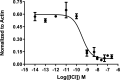
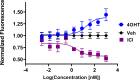
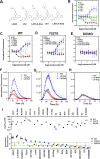
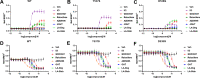
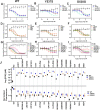
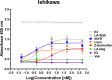
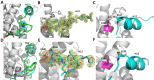
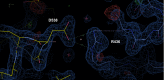
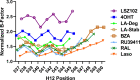
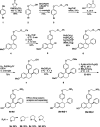
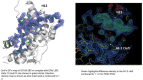
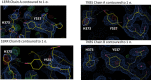
Chemical manipulation of estrogen receptor alpha ligand binding domain structural mobility tunes receptor lifetime and influences breast cancer therapeutic activities. Selective estrogen receptor modulators (SERMs) extend estrogen receptor alpha (ERα) cellular lifetime/accumulation. They are antagonists in the breast but agonists in the uterine epithelium and/or in bone. Selective estrogen receptor degraders/downregulators (SERDs) reduce ERα cellular lifetime/accumulation and are pure antagonists. Activating somatic ESR1 mutations Y537S and D538G enable resistance to first-line endocrine therapies. SERDs have shown significant activities in ESR1 mutant setting while few SERMs have been studied. To understand whether chemical manipulation of ERα cellular lifetime and accumulation influences antagonistic activity, we studied a series of methylpyrollidine lasofoxifene (Laso) derivatives that maintained the drug's antagonistic activities while uniquely tuning ERα cellular accumulation. These molecules were examined alongside a panel of antiestrogens in live cell assays of ERα cellular accumulation, lifetime, SUMOylation, and transcriptional antagonism. High-resolution x-ray crystal structures of WT and Y537S ERα ligand binding domain in complex with the methylated Laso derivatives or representative SERMs and SERDs show that molecules that favor a highly buried helix 12 antagonist conformation achieve the greatest transcriptional suppression activities in breast cancer cells harboring WT/Y537S ESR1. Together these results show that chemical reduction of ERα cellular lifetime is not necessarily the most crucial parameter for transcriptional antagonism in ESR1 mutated breast cancer cells. Importantly, our studies show how small chemical differences within a scaffold series can provide compounds with similar antagonistic activities, but with greatly different effects of the cellular lifetime of the ERα, which is crucial for achieving desired SERM or SERD profiles.
Keywords: D538G ESR1 Mutation; E. coli; Y537S ESR1 Mutation; antiestrogen; biochemistry; breast cancer; cancer biology; chemical biology; drug resistance; estrogen receptor degradation; hormone resistance; human.
© 2022, Hosfield et al.
Conflict of interest statement
DH, SW, NL, MS, CJ, GH, ES, EN, RH, SC, ML, SM, GG No competing interests declared, SF In the interest of transparency, Dr. Fanning's laboratory receives sponsored research funds from Olema Oncology Inc. Olema was not involved in this study. This work has no impact on the company
Figures






Similar articles
-
Targeting unique ligand binding domain structural features downregulates DKK1 in Y537S ESR1 mutant breast cancer cells.Breast Cancer Res. 2025 Jan 17;27(1):10. doi: 10.1186/s13058-024-01945-z. Breast Cancer Res. 2025. PMID: 39825366 Free PMC article.
-
Lasofoxifene as a potential treatment for therapy-resistant ER-positive metastatic breast cancer.Breast Cancer Res. 2021 May 12;23(1):54. doi: 10.1186/s13058-021-01431-w. Breast Cancer Res. 2021. PMID: 33980285 Free PMC article.
-
Mutation site and context dependent effects of ESR1 mutation in genome-edited breast cancer cell models.Breast Cancer Res. 2017 May 23;19(1):60. doi: 10.1186/s13058-017-0851-4. Breast Cancer Res. 2017. PMID: 28535794 Free PMC article.
-
The race to develop oral SERDs and other novel estrogen receptor inhibitors: recent clinical trial results and impact on treatment options.Cancer Metastasis Rev. 2022 Dec;41(4):975-990. doi: 10.1007/s10555-022-10066-y. Epub 2022 Oct 14. Cancer Metastasis Rev. 2022. PMID: 36229710 Free PMC article. Review.
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
Cited by
-
Elucidation of Pharmacological Mechanism Underlying the Anti-Alzheimer's Disease Effects of Evodia rutaecarpa and Discovery of Novel Lead Molecules: An In Silico Study.Molecules. 2023 Aug 3;28(15):5846. doi: 10.3390/molecules28155846. Molecules. 2023. PMID: 37570816 Free PMC article.
-
Development of new bioactive molecules to treat breast and lung cancer with natural myricetin and its derivatives: A computational and SAR approach.Front Cell Infect Microbiol. 2022 Sep 27;12:952297. doi: 10.3389/fcimb.2022.952297. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36237438 Free PMC article.
-
Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential.Int J Mol Sci. 2025 Jul 11;26(14):6668. doi: 10.3390/ijms26146668. Int J Mol Sci. 2025. PMID: 40724916 Free PMC article.
-
Network Pharmacology-Integrated Molecular Docking Reveals the Expected Anticancer Mechanism of Picrorhizae Rhizoma Extract.Biomed Res Int. 2022 Sep 16;2022:3268773. doi: 10.1155/2022/3268773. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2024 Jan 9;2024:9854052. doi: 10.1155/2024/9854052. PMID: 36158891 Free PMC article. Retracted.
-
In silico design of novel bioactive molecules to treat breast cancer with chlorogenic acid derivatives: a computational and SAR approach.Front Pharmacol. 2023 Dec 12;14:1266833. doi: 10.3389/fphar.2023.1266833. eCollection 2023. Front Pharmacol. 2023. PMID: 38152692 Free PMC article.
References
-
- Andreano KJ, Baker JG, Park S, Safi R, Artham S, Oesterreich S, Jeselsohn R, Brown M, Sammons S, Wardell SE, Chang CY, Norris JD, McDonnell DP. The Dysregulated Pharmacology of Clinically Relevant ESR1 Mutants is Normalized by Ligand-activated WT Receptor. Molecular Cancer Therapeutics. 2020;19:1395–1405. doi: 10.1158/1535-7163.MCT-19-1148. - DOI - PMC - PubMed
-
- Bardia A, Linden HM, Ulaner GA, Chandarlapaty S, Gosselin A, Doroumian S, Celanovic M, Campone M. Dose-escalation study of SAR439859, an oral selective estrogen receptor (ER) degrader (SERD), in postmenopausal women with ER+/HER2- metastatic breast cancer (mBC) Journal of Clinical Oncology. 2019;37:e1054. doi: 10.1200/JCO.2019.37.15_suppl.1054. - DOI
-
- Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. Journal of Clinical Oncology. 2019;37:423–438. doi: 10.1200/JCO.18.01160. - DOI - PubMed
-
- Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, Bhatt T, Patel P, Voi M, Gnant M, Hortobagyi G, Baselga J, Moynahan ME. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial. JAMA Oncology. 2016;2:1310–1315. doi: 10.1001/jamaoncol.2016.1279. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

