Drosophila nicotinic acetylcholine receptor subunits and their native interactions with insecticidal peptide toxins
- PMID: 35575460
- PMCID: PMC9110030
- DOI: 10.7554/eLife.74322
Drosophila nicotinic acetylcholine receptor subunits and their native interactions with insecticidal peptide toxins
Abstract
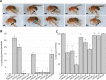
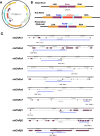
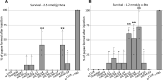
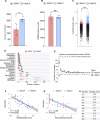
Drosophila nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels that represent a target for insecticides. Peptide neurotoxins are known to block nAChRs by binding to their target subunits, however, a better understanding of this mechanism is needed for effective insecticide design. To facilitate the analysis of nAChRs we used a CRISPR/Cas9 strategy to generate null alleles for all ten nAChR subunit genes in a common genetic background. We studied interactions of nAChR subunits with peptide neurotoxins by larval injections and styrene maleic acid lipid particles (SMALPs) pull-down assays. For the null alleles, we determined the effects of α-Bungarotoxin (α-Btx) and ω-Hexatoxin-Hv1a (Hv1a) administration, identifying potential receptor subunits implicated in the binding of these toxins. We employed pull-down assays to confirm α-Btx interactions with the Drosophila α5 (Dα5), Dα6, Dα7 subunits. Finally, we report the localisation of fluorescent tagged endogenous Dα6 during Drosophila CNS development. Taken together, this study elucidates native Drosophila nAChR subunit interactions with insecticidal peptide toxins and provides a resource for the in vivo analysis of insect nAChRs.
Keywords: D. melanogaster; Drosophila; Drosophila nAChRs; insecticidal toxins; neuroscience; neurotoxin interactions.
© 2022, Korona et al.
Conflict of interest statement
DK, CG, RQ, RP, KM, DM, MD, GJ, FE, SR, KL No competing interests declared, BD, LF is affiliated with Syngenta. The author has no other competing interests to declare
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

