Adenosine Awakens Metabolism to Enhance Growth-Independent Killing of Tolerant and Persister Bacteria across Multiple Classes of Antibiotics
- PMID: 35575513
- PMCID: PMC9239199
- DOI: 10.1128/mbio.00480-22
Adenosine Awakens Metabolism to Enhance Growth-Independent Killing of Tolerant and Persister Bacteria across Multiple Classes of Antibiotics
Abstract
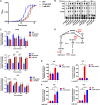
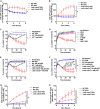
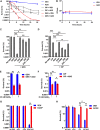
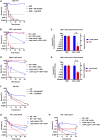
Metabolic and growth arrest are primary drivers of antibiotic tolerance and persistence in clinically diverse bacterial pathogens. We recently showed that adenosine (ADO) suppresses bacterial growth under nutrient-limiting conditions. In the current study, we show that despite the growth-suppressive effect of ADO, extracellular ADO enhances antibiotic killing in both Gram-negative and Gram-positive bacteria by up to 5 orders of magnitude. The ADO-potentiated antibiotic activity is dependent on purine salvage and is paralleled with a suppression of guanosine tetraphosphate synthesis and the massive accumulation of ATP and GTP. These changes in nucleoside phosphates coincide with transient increases in rRNA transcription and proton motive force. The potentiation of antibiotic killing by ADO is manifested against bacteria grown under both aerobic and anaerobic conditions, and it is exhibited even in the absence of alternative electron acceptors such as nitrate. ADO potentiates antibiotic killing by generating proton motive force and can occur independently of an ATP synthase. Bacteria treated with an uncoupler of oxidative phosphorylation and NADH dehydrogenase-deficient bacteria are refractory to the ADO-potentiated killing, suggesting that the metabolic awakening induced by this nucleoside is intrinsically dependent on an energized membrane. In conclusion, ADO represents a novel example of metabolite-driven but growth-independent means to reverse antibiotic tolerance. Our investigations identify the purine salvage pathway as a potential target for the development of therapeutics that may improve infection clearance while reducing the emergence of antibiotic resistance. IMPORTANCE Antibiotic tolerance, which is a hallmark of persister bacteria, contributes to treatment-refractory infections and the emergence of heritable antimicrobial resistance. Drugs that reverse tolerance and persistence may become part of the arsenal to combat antimicrobial resistance. Here, we demonstrate that salvage of extracellular ADO reduces antibiotic tolerance in nutritionally stressed Escherichia coli, Salmonella enterica, and Staphylococcus aureus. ADO potentiates bacterial killing under aerobic and anaerobic conditions and takes place in bacteria lacking the ATP synthase. However, the sensitization to antibiotic killing elicited by ADO requires an intact NADH dehydrogenase, suggesting a requirement for an energized electron transport chain. ADO antagonizes antibiotic tolerance by activating ATP and GTP synthesis, promoting proton motive force and cellular respiration while simultaneously suppressing the stringent response. These investigations reveal an unprecedented role for purine salvage stimulation as a countermeasure of antibiotic tolerance and the emergence of antimicrobial resistance.
Keywords: E. coli; Salmonella; antibiotic resistance; nucleosides; persistence; tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- IK6 BX005384/BX/BLRD VA/United States
- R01 AI136520/AI/NIAID NIH HHS/United States
- R56 AI054959/AI/NIAID NIH HHS/United States
- T32 AI052066/AI/NIAID NIH HHS/United States
- R01 DK095491/DK/NIDDK NIH HHS/United States
- R01 DK104713/DK/NIDDK NIH HHS/United States
- P30 DK048520/DK/NIDDK NIH HHS/United States
- K01 DK129410/DK/NIDDK NIH HHS/United States
- K08 DK120809/DK/NIDDK NIH HHS/United States
- I01 BX002073/BX/BLRD VA/United States
- R01 AI155493/AI/NIAID NIH HHS/United States
- R01 AI054959/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
