Blocking Polyphosphate Mobilization Inhibits Pho4 Activation and Virulence in the Pathogen Candida albicans
- PMID: 35575514
- PMCID: PMC9239153
- DOI: 10.1128/mbio.00342-22
Blocking Polyphosphate Mobilization Inhibits Pho4 Activation and Virulence in the Pathogen Candida albicans
Abstract
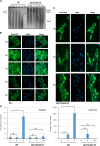
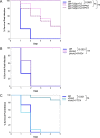
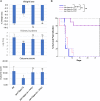
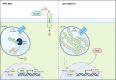
The ability of pathogenic fungi to obtain essential nutrients from the host is vital for virulence. In Candida albicans, acquisition of the macronutrient phosphate is regulated by the Pho4 transcription factor and is important for both virulence and resistance to host-encountered stresses. All cells store phosphate in the form of polyphosphate (polyP), a ubiquitous polymer comprising tens to hundreds of phosphate residues. Release of phosphate from polyP is one of the first responses evoked in response to phosphate starvation, and here, we sought to explore the importance of polyP mobilization in the pathobiology of C. albicans. We found that two polyphosphatases, Ppn1 and Ppx1, function redundantly to release phosphate from polyP in C. albicans. Strikingly, we reveal that blocking polyP mobilization prevents the activation of the Pho4 transcription factor: following Pi starvation, Pho4 fails to accumulate in the nucleus and induce Pi acquisition genes in ppn1Δ ppx1Δ cells. Consequently, ppn1Δ ppx1Δ cells display impaired resistance to the same range of stresses that require Pho4 for survival. In addition, cells lacking both polyphosphatases are exquisitely sensitive to DNA replication stress, indicating that polyP mobilization is needed to support the phosphate-demanding process of DNA replication. Blocking polyP mobilization also results in significant morphological defects, as ppn1Δ ppx1Δ cells form large pseudohypha-like cells that are resistant to serum-induced hypha formation. Thus, polyP mobilization impacts key processes important for the pathobiology of C. albicans, and consistent with this, we found that blocking this process attenuates the virulence of this important human fungal pathogen. IMPORTANCE Acquisition of the essential macronutrient phosphate is important for the virulence of Candida albicans, a major human fungal pathogen. All cells store phosphate as polyphosphate (polyP), which is rapidly mobilized when phosphate is limiting. Here, we identified the major phosphatases involved in releasing phosphate from polyP in C. albicans. By blocking this process, we found that polyP mobilization impacts many process that contribute to C. albicans pathogenesis. Notably, we found that blocking polyP mobilization inhibits activation of the Pho4 transcription factor, the master regulator of phosphate acquisition. In addition, cell cycle progression, stress resistance, morphogenetic switching, and virulence are all impaired in cells that cannot mobilize polyP. This study therefore provides new insight into the importance of polyP mobilization in promoting the virulence of C. albicans. As phosphate homeostasis strategies differ between fungal pathogen and host, this offers promise for the future development of antifungals.
Keywords: Candida albicans; morphogenesis; phosphate metabolism; stress response; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis.Mol Biol Cell. 2016 Sep 1;27(17):2784-801. doi: 10.1091/mbc.E16-05-0266. Epub 2016 Jul 6. Mol Biol Cell. 2016. PMID: 27385340 Free PMC article.
-
Phosphate Acquisition and Virulence in Human Fungal Pathogens.Microorganisms. 2017 Aug 22;5(3):48. doi: 10.3390/microorganisms5030048. Microorganisms. 2017. PMID: 28829379 Free PMC article. Review.
-
Genetic Analysis of NDT80 Family Transcription Factors in Candida albicans Using New CRISPR-Cas9 Approaches.mSphere. 2018 Nov 21;3(6):e00545-18. doi: 10.1128/mSphere.00545-18. mSphere. 2018. PMID: 30463924 Free PMC article.
-
A highly conserved tRNA modification contributes to C. albicans filamentation and virulence.Microbiol Spectr. 2024 May 2;12(5):e0425522. doi: 10.1128/spectrum.04255-22. Epub 2024 Apr 8. Microbiol Spectr. 2024. PMID: 38587411 Free PMC article.
-
Co-regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen.Int J Med Microbiol. 2002 Oct;292(5-6):299-311. doi: 10.1078/1438-4221-00215. Int J Med Microbiol. 2002. PMID: 12452278 Review.
Cited by
-
Polyphosphatases have a polyphosphate-independent influence on the virulence of Cryptococcus neoformans.Infect Immun. 2025 Apr 8;93(4):e0007225. doi: 10.1128/iai.00072-25. Epub 2025 Mar 12. Infect Immun. 2025. PMID: 40071953 Free PMC article.
-
A transcriptional activator from Rhizophagus irregularis regulates phosphate uptake and homeostasis in AM symbiosis during phosphorous starvation.Front Microbiol. 2023 Jan 20;13:1114089. doi: 10.3389/fmicb.2022.1114089. eCollection 2022. Front Microbiol. 2023. PMID: 36741887 Free PMC article.
References
-
- Ikeh MAC, Kastora SL, Day AM, Herrero-de-Dios CM, Tarrant E, Waldron KJ, Banks AP, Bain JM, Lydall D, Veal EA, MacCallum DM, Erwig LP, Brown AJP, Quinn J. 2016. Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis. Mol Biol Cell 27:2784–2801. doi:10.1091/mbc.e16-05-0266. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
