Lifetime medical costs attributable to sickle cell disease among nonelderly individuals with commercial insurance
- PMID: 35575558
- PMCID: PMC9898623
- DOI: 10.1182/bloodadvances.2021006281
Lifetime medical costs attributable to sickle cell disease among nonelderly individuals with commercial insurance
Abstract
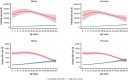
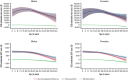
Sickle cell disease (SCD) is a severe monogenic disease associated with high morbidity, mortality, and a disproportionate burden on Black and Hispanic communities. Our objective was to estimate the total healthcare costs and out-of-pocket (OOP) costs attributable to SCD among commercially insured individuals over their nonelderly lifetimes (0 to 64 years of age). We constructed a retrospective cohort of individuals with diagnosed SCD using Truven Health Marketscan commercial claims data from 2007 through 2018, compared with matched control subjects from the Medical Expenditure Panel Survey. We estimated Kaplan-Meier sample average costs using previously reported survival curves for SCD and control subjects. Individuals with SCD (20 891) and control subjects (33 588) were included in our analysis. The SCD sample had a mean age of 25.7 (standard deviation, 17.4) years; 58.0% were female. Survival-adjusted costs of SCD peaked at age 13 to 24 years and declined at older ages. There was no significant difference in total medical costs or OOP costs between the sexes. SCD-attributable costs over 0 to 64 years of age were estimated to be $1.6 million (95% confidence interval [CI], $1.3M-$1.9M) and $1.7 million (95% CI, $1.4M-$2.1M) for females and males with SCD, respectively. The corresponding OOP estimates were $42 395 (95% CI, $34 756-$50 033) for females and $45 091 (95% CI, $36 491-$53 691) for males. These represent a 907% and 285% increase in total medical and OOP costs over control subjects, respectively. Although limited to the commercially insured population, these results indicate that the direct economic burden of SCD is substantial and peaks at younger ages, suggesting the need for curative and new medical therapies.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Centers for Disease Control and Prevention Data & statistics on sickle cell disease. www.cdc.gov/ncbddd/sicklecell/data.html
-
- Fingar KR, Owens PL, Reid LD, et al. Healthcare cost and utilization project (hcup) statistical briefs. Agency for Healthcare Research and Quality; 2019. Characteristics of inpatient hospital stays involving sickle cell disease, 2000–2016: statistical brief #251.http://www.ncbi.nlm.nih.gov/books/NBK547764/ - PubMed
-
- Bou-Maroun LM, Meta F, Hanba CJ, Campbell AD, Yanik GA. An analysis of inpatient pediatric sickle cell disease: Incidence, costs, and outcomes. Pediatr Blood Cancer. 2017;65(1) - PubMed
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. - PubMed
-
- Blinder MA, Duh MS, Sasane M, Trahey A, Paley C, Vekeman F. Age-related emergency department reliance in patients with sickle cell disease. J Emerg Med. 2015;49(4):513–522.e1. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

