Multicenter Population Pharmacokinetic Study of Unbound Ceftriaxone in Critically Ill Patients
- PMID: 35575578
- PMCID: PMC9211414
- DOI: 10.1128/aac.02189-21
Multicenter Population Pharmacokinetic Study of Unbound Ceftriaxone in Critically Ill Patients
Abstract
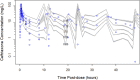
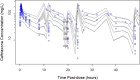
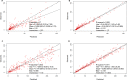
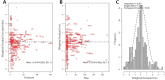
The objective of this study was to describe the total and unbound population pharmacokinetics of ceftriaxone in critically ill adult patients and to define optimized dosing regimens. Total and unbound ceftriaxone concentrations were obtained from two pharmacokinetic studies and from a therapeutic drug monitoring (TDM) program at a tertiary hospital intensive care unit. Population pharmacokinetic analysis and Monte Carlo simulations were used to assess the probability of achieving a free trough concentration/MIC ratio of ≥1 using Pmetrics for R. A total of 474 samples (267 total and 207 unbound) were available from 36 patients. A two-compartment model describing ceftriaxone-albumin binding with both nonrenal and renal elimination incorporating creatinine clearance to explain the between-patient variability best described the data. An albumin concentration of ≤20 g/L decreased the probability of target attainment (PTA) by up to 20% across different dosing regimens and simulated creatinine clearances. A ceftriaxone dose of 1 g twice daily is likely therapeutic in patients with creatinine clearance of <100 mL/min infected with susceptible isolates (PTA, ~90%). Higher doses administered as a continuous infusion (4 g/day) are needed in patients with augmented renal clearance (creatinine clearance, >130 mL/min) who are infected by pathogens with a MIC of ≥0.5 mg/L. The ceftriaxone dose should be based on the patient's renal function and albumin concentration, as well as the isolate MIC. Hypoalbuminemia decreases the PTA in patients receiving intermittent dosing by up to 20%.
Keywords: ceftriaxone; dose; intensive care; intensive care unit; pharmacodynamics; pharmacokinetics; population pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Abdul-Aziz MH, Alffenaar J-WC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely MN, Paiva J-A, Pea F, Sjovall F, Timsit JF, Udy AA, Wicha SG, Zeitlinger M, De Waele JJ, Roberts JA, the Infection Section of European Society of Intensive Care Medicine (ESICM) . 2020. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 46:1127–1153. 10.1007/s00134-020-06050-1. - DOI - PMC - PubMed
-
- McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. 10.1016/j.ijantimicag.2007.12.009. - DOI - PubMed
-
- Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen K-M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Roberts JA, Lipman J, Starr T, Wallis SC, Paul SK, Margarit Ribas A, De Waele JJ, De Crop L, Spapen H, Wauters J, Dugernier T, Jorens P, Dapper I, De Backer D, Taccone FS, Rello J, Ruano L, Afonso E, Alvarez-Lerma F, Gracia-Arnillas MP, Fernandez F, Feijoo N, Bardolet N, Rovira A, Garro P, Colon D, Castillo C, Fernado J, Lopez MJ, Fernandez JL, Arribas AM, Teja JL, Ots E, Carlos Montejo J, Catalan M, et al. . 2014. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. 10.1093/cid/ciu027. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

