Prenatal choline supplementation improves biomarkers of maternal docosahexaenoic acid (DHA) status among pregnant participants consuming supplemental DHA: a randomized controlled trial
- PMID: 35575618
- PMCID: PMC9437984
- DOI: 10.1093/ajcn/nqac147
Prenatal choline supplementation improves biomarkers of maternal docosahexaenoic acid (DHA) status among pregnant participants consuming supplemental DHA: a randomized controlled trial
Abstract
Background: Dietary methyl donors (e.g., choline) support the activity of the phosphatidylethanolamine N-methyltransferase (PEMT) pathway, which generates phosphatidylcholine (PC) molecules enriched in DHA that are exported from the liver and made available to extrahepatic tissues.
Objectives: This study investigated the effect of prenatal choline supplementation on biomarkers of DHA status among pregnant participants consuming supplemental DHA.
Methods: Pregnant participants (n = 30) were randomly assigned to receive supplemental choline intakes of 550 mg/d [500 mg/d d0-choline + 50 mg/d deuterium-labeled choline (d9-choline); intervention] or 25 mg/d (25 mg/d d9-choline; control) from gestational week (GW) 12-16 until delivery. All participants received a daily 200-mg DHA supplement and consumed self-selected diets. Fasting blood samples were obtained at baseline, GW 20-24, and GW 28-32; maternal/cord blood was obtained at delivery. Mixed-effects linear models were used to assess the impact of prenatal choline supplementation on maternal and newborn DHA status.
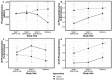
Results: Choline supplementation (550 vs. 25 mg/d) did not achieve a statistically significant intervention × time interaction for RBC PC-DHA (P = 0.11); a significant interaction was observed for plasma PC-DHA and RBC total DHA, with choline supplementation yielding higher levels (+32-38% and +8-11%, respectively) at GW 28-32 (P < 0.05) and delivery (P < 0.005). A main effect of choline supplementation on plasma total DHA was also observed (P = 0.018); its interaction with time was not significant (P = 0.068). Compared with controls, the intervention group exhibited higher (P = 0.007; main effect) plasma enrichment of d3-PC (d3-PC/total PC). Moreover, the ratio of d3-PC to d9-PC was higher (+50-67%; P < 0.001) in the choline intervention arm (vs. control) at GW 20-24, GW 28-32, and delivery.
Conclusions: Prenatal choline supplementation improves hepatic DHA export and biomarkers of DHA status by bolstering methyl group supply for PEMT activity among pregnant participants consuming supplemental DHA. This trial is registered at www.clinicaltrials.gov as NCT03194659.
Keywords: PEMT pathway; docosahexaenoic acid; omega-3 polyunsaturated fatty acids; pregnancy; prenatal choline supplementation; stable isotope.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures

References
-
- Brenna JT, Lapillonne A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab. 2009;55(1-3):97–122. - PubMed
-
- Koletzko B, Cetin I, Brenna JT Perinatal Lipid Intake Working Group, Child Health Foundation, Diabetic Pregnancy Study Group; et al. .; Perinatal Lipid Intake Working Group, Child Health Foundation, Diabetic Pregnancy Study Group . Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98(5):873–7. - PubMed
-
- Watkins SM, Zhu X, Zeisel SH. Phosphatidylethanolamine-N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J Nutr. 2003;133(11):3386–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

