Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments
- PMID: 35575644
- PMCID: PMC9332215
- DOI: 10.1039/d0cs01079k
Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments
Abstract
Replacing fossil fuels with energy sources and carriers that are sustainable, environmentally benign, and affordable is amongst the most pressing challenges for future socio-economic development. To that goal, hydrogen is presumed to be the most promising energy carrier. Electrocatalytic water splitting, if driven by green electricity, would provide hydrogen with minimal CO2 footprint. The viability of water electrolysis still hinges on the availability of durable earth-abundant electrocatalyst materials and the overall process efficiency. This review spans from the fundamentals of electrocatalytically initiated water splitting to the very latest scientific findings from university and institutional research, also covering specifications and special features of the current industrial processes and those processes currently being tested in large-scale applications. Recently developed strategies are described for the optimisation and discovery of active and durable materials for electrodes that ever-increasingly harness first-principles calculations and machine learning. In addition, a technoeconomic analysis of water electrolysis is included that allows an assessment of the extent to which a large-scale implementation of water splitting can help to combat climate change. This review article is intended to cross-pollinate and strengthen efforts from fundamental understanding to technical implementation and to improve the 'junctions' between the field's physical chemists, materials scientists and engineers, as well as stimulate much-needed exchange among these groups on challenges encountered in the different domains.
Conflict of interest statement
There are no conflicts to declare.
Figures



















































































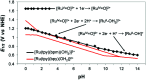

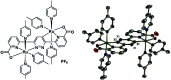

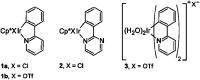

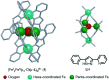







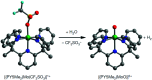
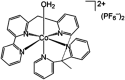




















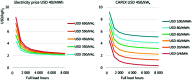

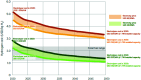







References
-
- “Sir David Attenborough: We Must Act on Population”. Population Matters. 5 October 2018. Retrieved 1 December 2019
-
- Poizot P. Dolhelm F. Energy Environ. Sci. 2011;4:2003–2019. doi: 10.1039/C0EE00731E. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

