Modeling the Effects of Disease, Drug Properties, and Material on Drug Transport From Intraocular Lenses
- PMID: 35575775
- PMCID: PMC9123490
- DOI: 10.1167/tvst.11.5.14
Modeling the Effects of Disease, Drug Properties, and Material on Drug Transport From Intraocular Lenses
Abstract
Purpose: Surgically implanted intraocular lenses (IOLs) may be used as drug-delivery devices, but their effectiveness is not well defined. Computational fluid dynamics models were developed to investigate the capability of IOLs to release drugs at therapeutic concentrations.
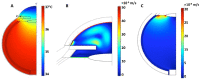
Methods: Models were generated using COMSOL Multiphysics. Primary open-angle glaucoma (POAG) and wet age-related macular degeneration (AMD) were simulated by reducing aqueous vein and choroidal blood flow, respectively. Release of dexamethasone, ganciclovir, or dextran was studied using common IOL materials, polydimethylsiloxane (PDMS) and poly(2-hydroxyethyl methacrylate) (PHEMA).
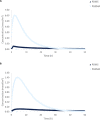
Results: Drug clearance proceeds mainly through choroidal blood flow. When fully constricted, maximum concentration at the choroid (Cmax) values increased by 32.4% to 39,800%. Compared to dexamethasone, Cmax in different tissues decreased by 6.07% to 96.0% for ganciclovir and dextran, and clearance rates decreased by 16% to 69% for ganciclovir and by 92% to 100% for dextran. Using PDMS as the IOL reduced clearance rates by 91.3% to 94.6% compared to PHEMA.
Conclusions: In diseased eyes, drugs accumulate mainly in posterior tissue; thus, choroidal drug toxicity must be assessed prior to IOL implantation in POAG and AMD patients. Moreover, drug properties modulated concentration profiles in all ocular segments. The hydrophobic small-molecule dexamethasone attained the highest concentrations and cleared the fastest, whereas hydrophilic macromolecular dextran attained the lowest concentrations and cleared the slowest. Furthermore, high concentrations were achieved quickly following release from PHEMA, whereas PDMS allowed for sustained release.
Translational relevance: In silico results can guide scientists and clinicians regarding important physiological and chemical factors that modulate tissue drug concentrations from drug-eluting IOLs.
Conflict of interest statement
Disclosure:
Figures




Similar articles
-
Topography, Wettability, and Electrostatic Charge Consist Major Surface Properties of Intraocular Lenses.Curr Eye Res. 2017 Feb;42(2):201-210. doi: 10.3109/02713683.2016.1164187. Epub 2016 Aug 22. Curr Eye Res. 2017. PMID: 27548409
-
Dual drug delivery from hydrophobic and hydrophilic intraocular lenses: in-vitro and in-vivo studies.J Control Release. 2020 Oct 10;326:245-255. doi: 10.1016/j.jconrel.2020.07.020. Epub 2020 Jul 16. J Control Release. 2020. PMID: 32682901
-
Cyclodextrin-containing hydrogels as an intraocular lens for sustained drug release.PLoS One. 2017 Dec 15;12(12):e0189778. doi: 10.1371/journal.pone.0189778. eCollection 2017. PLoS One. 2017. PMID: 29244868 Free PMC article.
-
Intraocular lenses as drug delivery devices.Int J Pharm. 2021 Jun 1;602:120613. doi: 10.1016/j.ijpharm.2021.120613. Epub 2021 Apr 16. Int J Pharm. 2021. PMID: 33865952 Review.
-
[Studies on clinical pathophysiology of pseudophakic/aphakic eyes--a journey of 4 decades].Nippon Ganka Gakkai Zasshi. 2008 Mar;112(3):214-45; discussion 246. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18411712 Review. Japanese.
Cited by
-
Investigating recent advances in pharmacotherapy for acute and chronic ocular pain post-cataract surgery.Expert Opin Pharmacother. 2024 Nov;25(16):2107-2113. doi: 10.1080/14656566.2024.2421323. Epub 2024 Oct 28. Expert Opin Pharmacother. 2024. PMID: 39441206 No abstract available.
References
-
- Steinert R, Jain R.. Ophthalmologic applications: introduction. In: Ratner B, Hoffman A, Schoen F, Lemons J, eds. Biomaterials Science: An Introduction to Materials in Medicine. 3rd ed. Amsterdam: Elsevier; 2013: 905–956.
-
- Jürgens I. Secondary cataract. Available at: https://icrcat.com/en/eye-conditions/secondary-cataract/. Accessed November 2, 2020.
-
- Liu Y, Wong TT, Mehta JS.. Intraocular lens as a drug delivery reservoir. Curr Opin Ophthalmol. 2013; 24(1): 53–59. - PubMed
-
- National Eye Institute. Uveitis. Available at: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseas.... Accessed September 18, 2020.
-
- Thorne J, Suhler E, Skup M.. Prevalence of uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016; 134(11): 1237–1245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

