Prediction of Retinal Ganglion Cell Counts Considering Various Displacement Methods From OCT-Derived Ganglion Cell-Inner Plexiform Layer Thickness
- PMID: 35575777
- PMCID: PMC9123515
- DOI: 10.1167/tvst.11.5.13
Prediction of Retinal Ganglion Cell Counts Considering Various Displacement Methods From OCT-Derived Ganglion Cell-Inner Plexiform Layer Thickness
Abstract
Purpose: To compare various displacement models using midget retinal ganglion cell to cone (mRGC:C) ratios and to determine viability of estimating RGC counts from optical coherence tomography (OCT)-derived ganglion cell-inner plexiform layer (GCIPL) measurements.
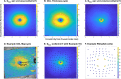
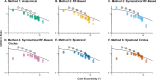
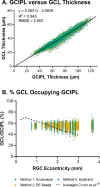
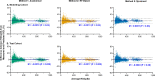
Methods: Four Drasdo model variations were applied to macular visual field (VF) stimulus locations: (1) using meridian-specific Henle fiber length along the stimulus circumference; (2) using meridian-specific differences in RGC receptive field and counts along the stimulus circumference; (3) per method (2), averaged across principal meridians; and (4) per method (3), with the stimulus center displaced only. The Sjöstrand model was applied (5) along the stimulus circumference and (6) to the stimulus center only. Eccentricity-dependent mRGC:C ratios were computed over displaced areas, with comparisons to previous models using sum of squares of the residuals (SSR) and root mean square error (RMSE). RGC counts estimated from OCT-derived ganglion cell layer (GCL) and GCIPL measurements, from 143 healthy participants, were compared using Bland-Altman analyses.
Results: Methods 1, 2, and 5 produced mRGC:C ratios most consistent with previous models (SSR 3.82, 4.07, and 3.02; RMSE 0.22, 0.23, and 0.20), while central mRGC:C ratios were overestimated by method 3 and underestimated by methods 4 and 6. RGC counts predicted from GCIPL measurements were within 16% of GCL-based counts, with no notable bias with increasing RGC counts.
Conclusions: Sjöstrand displacement and meridian-specific Drasdo displacement applied to VF stimulus circumferences produce mRGC:C ratios consistent with previous models. RGC counts can be estimated from OCT-derived GCIPL measurements.
Translational relevance: Implementing appropriate displacement methods and deriving RGC estimates from relevant OCT parameters enables calculation of the number of RGCs responding to VF stimuli from commercial instrumentation.
Conflict of interest statement
Disclosure:
Figures


Similar articles
-
Ganglion cell-inner plexiform layer measurements derived from widefield compared to montaged 9-field optical coherence tomography.Clin Exp Optom. 2022 Nov;105(8):822-830. doi: 10.1080/08164622.2021.1993058. Epub 2021 Nov 18. Clin Exp Optom. 2022. PMID: 34791988
-
Custom extraction of macular ganglion cell-inner plexiform layer thickness more precisely co-localizes structural measurements with visual fields test grids.Sci Rep. 2020 Oct 28;10(1):18527. doi: 10.1038/s41598-020-75599-0. Sci Rep. 2020. PMID: 33116253 Free PMC article.
-
Revisiting the Drasdo Model: Implications for Structure-Function Analysis of the Macular Region.Transl Vis Sci Technol. 2020 Sep 14;9(10):15. doi: 10.1167/tvst.9.10.15. eCollection 2020 Sep. Transl Vis Sci Technol. 2020. PMID: 32974087 Free PMC article.
-
Macular ganglion cell-inner plexiform layer thinning in patients with visual field defect that respects the vertical meridian.Graefes Arch Clin Exp Ophthalmol. 2014 Sep;252(9):1501-7. doi: 10.1007/s00417-014-2706-3. Epub 2014 Aug 9. Graefes Arch Clin Exp Ophthalmol. 2014. PMID: 25104464
-
Glaucomatous damage of the macula.Prog Retin Eye Res. 2013 Jan;32:1-21. doi: 10.1016/j.preteyeres.2012.08.003. Epub 2012 Sep 17. Prog Retin Eye Res. 2013. PMID: 22995953 Free PMC article. Review.
Cited by
-
High sampling resolution optical coherence tomography reveals potential concurrent reductions in ganglion cell-inner plexiform and inner nuclear layer thickness but not in outer retinal thickness in glaucoma.Ophthalmic Physiol Opt. 2023 Jan;43(1):46-63. doi: 10.1111/opo.13065. Epub 2022 Nov 23. Ophthalmic Physiol Opt. 2023. PMID: 36416369 Free PMC article.
-
Spatial Summation in the Glaucomatous Macula: A Link With Retinal Ganglion Cell Damage.Invest Ophthalmol Vis Sci. 2023 Nov 1;64(14):36. doi: 10.1167/iovs.64.14.36. Invest Ophthalmol Vis Sci. 2023. PMID: 38010697 Free PMC article.
-
Author Response: Expected Improvement in Structure-Function Agreement With Macular Displacement Models.Transl Vis Sci Technol. 2022 Oct 3;11(10):15. doi: 10.1167/tvst.11.10.15. Transl Vis Sci Technol. 2022. PMID: 36219161 Free PMC article. No abstract available.
-
Letter to the Editor: Expected Improvement in Structure-Function Agreement With Macular Displacement Models.Transl Vis Sci Technol. 2022 Oct 3;11(10):14. doi: 10.1167/tvst.11.10.14. Transl Vis Sci Technol. 2022. PMID: 36219162 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

