Deactivation of the antiviral state by rabies virus through targeting and accumulation of persistently phosphorylated STAT1
- PMID: 35576230
- PMCID: PMC9135343
- DOI: 10.1371/journal.ppat.1010533
Deactivation of the antiviral state by rabies virus through targeting and accumulation of persistently phosphorylated STAT1
Abstract
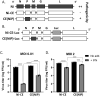
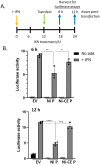
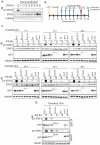
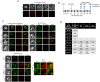
Antagonism of the interferon (IFN)-mediated antiviral state is critical to infection by rabies virus (RABV) and other viruses, and involves interference in the IFN induction and signaling pathways in infected cells, as well as deactivation of the antiviral state in cells previously activated by IFN. The latter is required for viral spread in the host, but the precise mechanisms involved and roles in RABV pathogenesis are poorly defined. Here, we examined the capacity of attenuated and pathogenic strains of RABV that differ only in the IFN-antagonist P protein to overcome an established antiviral state. Importantly, P protein selectively targets IFN-activated phosphorylated STAT1 (pY-STAT1), providing a molecular tool to elucidate specific roles of pY-STAT1. We find that the extended antiviral state is dependent on a low level of pY-STAT1 that appears to persist at a steady state through ongoing phosphorylation/dephosphorylation cycles, following an initial IFN-induced peak. P protein of pathogenic RABV binds and progressively accumulates pY-STAT1 in inactive cytoplasmic complexes, enabling recovery of efficient viral replication over time. Thus, P protein-pY-STAT1 interaction contributes to 'disarming' of the antiviral state. P protein of the attenuated RABV is defective in this respect, such that replication remains suppressed over extended periods in cells pre-activated by IFN. These data provide new insights into the nature of the antiviral state, indicating key roles for residual pY-STAT1 signaling. They also elucidate mechanisms of viral deactivation of antiviral responses, including specialized functions of P protein in selective targeting and accumulation of pY-STAT1.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: G.W.M. holds a patent application (PCT/AU2019/050908) and Australian provisional patent (No. 201901137); ‘‘Novel Viruses’’ 2019.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

