Telaglenastat plus Everolimus in Advanced Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled, Phase II ENTRATA Trial
- PMID: 35576438
- PMCID: PMC10202043
- DOI: 10.1158/1078-0432.CCR-22-0061
Telaglenastat plus Everolimus in Advanced Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled, Phase II ENTRATA Trial
Abstract
Purpose: Glutaminase is a key enzyme, which supports elevated dependency of tumors on glutamine-dependent biosynthesis of metabolic intermediates. Dual targeting of glucose and glutamine metabolism by the mTOR inhibitor everolimus plus the oral glutaminase inhibitor telaglenastat showed preclinical synergistic anticancer effects, which translated to encouraging safety and efficacy findings in a phase I trial of 2L+ renal cell carcinoma (RCC). This study evaluated telaglenastat plus everolimus (TelaE) versus placebo plus everolimus (PboE) in patients with advanced/metastatic RCC (mRCC) in the 3L+ setting (NCT03163667).
Patients and methods: Eligible patients with mRCC, previously treated with at least two prior lines of therapy [including ≥1 VEGFR-targeted tyrosine kinase inhibitor (TKI)] were randomized 2:1 to receive E, plus Tela or Pbo, until disease progression or unacceptable toxicity. Primary endpoint was investigator-assessed progression-free survival (PFS; one-sided α <0.2).
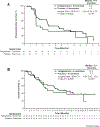
Results: Sixty-nine patients were randomized (46 TelaE, 23 PboE). Patients had a median three prior lines of therapy, including TKIs (100%) and checkpoint inhibitors (88%). At median follow-up of 7.5 months, median PFS was 3.8 months for TelaE versus 1.9 months for PboE [HR, 0.64; 95% confidence interval (CI), 0.34-1.20; one-sided P = 0.079]. One TelaE patient had a partial response and 26 had stable disease (SD). Eleven patients on PboE had SD. Treatment-emergent adverse events included fatigue, anemia, cough, dyspnea, elevated serum creatinine, and diarrhea; grade 3 to 4 events occurred in 74% TelaE patients versus 61% PboE.
Conclusions: TelaE was well tolerated and improved PFS versus PboE in patients with mRCC previously treated with TKIs and checkpoint inhibitors.
©2022 American Association for Cancer Research.
Conflict of interest statement
CHL has served as a consultant for Amgen, BMS, Exelixis, Eisai, Merck, Pfizer, and EMD Serono; received honoraria from AiCME, Intellisphere, and Research to Practice; and has received institutional research funding from BMS, Calithera, Eisai, Eli Lilly, Exelixis, Merck, and Pfizer.
RM has served on advisory boards for AstraZeneca, Aveo Pharmaceuticals, Calithera Biosciences, Eisai, EMD Serono, Exelixis, Genentech/Roche, Incyte, Lilly Oncology, Merck, Novartis, and Pfizer; and has received institutional research funding for clinical trials from Bristol Myers Squibb, Eisai, Exelixis, Genentech/Roche, Merck, and Pfizer.
HE has served as an advisory board consultant for Exelixis, Bayer, Bristol Myers Squibb, Seattle Genetics, and Cardinal Health; and has received institutional research funding from Bristol Myers Squibb, Exelixis, RepIimune, Merck, Calithera, Roche/Genentech, Janssen, and Arcus Biosciences.
MM has served as a consultant for Strata Oncology, AstraZeneca, and Dispersol; and on Speakers’ Bureaus for AstraZeneca, Merck, Bristol Myers Squibb, Astellas, Eisai, Janssen, and SeaGen.
JH has received honoraria for speaker, consultancy, or advisory roles from Eisai and BostonGene, and research funding from Merck, BostonGene, TScan, Bristol Myers Squibb, AstraZeneca, Exelixis, Calithera, and SillaJen.
UV has received honoraria for speaker, consultancy, or advisory roles from Bristol Myers Squibb, Exelixis, EMD Serono, Eisai, AAA, Bayer, Alkermes, Sanofi, Genentech, and Merck.
SL has received honoraria for speaker, consultancy, or advisory roles from Exelixis, Merck, Eisai, Astellas, Seagen, Genentech, and Sanofi.
SM received research support for the ENTRATA study from Calithera Biosciences.
JCB has received institutional research funding from Gilead, Genentech/Roche, BMS, Five Prime, Lilly, Merck, MedImmune, Celgene, EMD Serono, Taiho, Macrogenics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Koltan, SynDevRex, Forty Seven, AbbVie, Array, Onyx, Sanofi, Takeda, Eisai, Celldex, Agios, Cytomx, Nektar, ARMO, Boston Biomedical, Ipsen, Merrimack, Tarveda, Tyrogenex, Oncogenex, Marshall Edwards, Pieris, Mersana, Calithera, Blueprint, Evelo, FORMA, Merus, Jacobio, Effector, Novocare, Arrys, Tracon, Sierra, Innate, Arch Oncology, Prelude Oncology, Unum Therapeutics, Vyriad, Harpoon, ADC, Amgen, Pfizer, Millennium, Imclone, Acerta Pharma, Rgenix, Bellicum, Gossamer Bio, Arcus Bio, Seattle Genetics, TempestTx, Shattuck Labs, Synthorx, Inc, Revolution Medicines, Inc., Bicycle Therapeutics, Zymeworks, Relay Therapeutics, Scholar Rock, NGM Biopharma, Stemcentrx, Beigene, CALGB, Cyteir Therapeutics, Foundation Bio, Innate Pharma, Morphotex, OncXerna, NuMab, AtlasMedx, Treadwell Therapeutics, IGM Biosciences, Mabspace, Hutchinson MediPharma, REPARE Therapeutics, NeoImmune Tech, Regeneron, and PureTech Health; consulting/advisory roles (institutional) for Gilead, Genentech / Roche, BMS, Five Prime, Lilly, Merck, MedImmune, Celgene, Taiho, Macrogenics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Array, Sanofi, ARMO, Ipsen, Merrimack, Oncogenex, FORMA, Arch Oncology, Prelude Therapeutics, Phoenix Bio, Cyteir, Molecular Partners, Innate, Torque, Tizona, Janssen, Tolero, Amgen, Seattle Genetics, Moderna Therapeutics, Tanabe Research Laboratories, Beigene, Continuum Clinical, Agios, Bicycle Therapeutics, Relay Therapeutics, Evelo, Pfizer, Samsung Bioepios, Fusion Therapeutics; and travel/food/beverage from Gilead, Genentech/Roche, BMS, Lilly, Merck, MedImmune, Celgene, Taiho, Novartis, OncoMed, BI, ARMO, Ipsen, Oncogenex, FORMA
ACF has consulted for Duke Clinical Research Institute (Verily Baseline Study), has served on an advisory board for Dendreon Inc, has received honoraria from the Medical Educator Consortium, has founder’s equity in Molecular Decisions, Inc., and has received institutional research funding from NCI, DOD, Conquer Cancer Foundation, Calithera Biosciences, Filtricine Inc., Earli Inc, and Vortex Biosciences.
OG has served on advisory boards for Bayer, Exelixis, and Janssen; and has participated on Speakers’ Bureaus for Janssen.
ARK has held consulting/advisory roles for Exelixis, AstraZeneca, Bayer, Pfizer, Novartis, Genentech, Bristol-Myers Squibb, and EMD Serono; participated in Speakers’ Bureaus for Janssen, Astellas Medivation, Pfizer, Novartis, Sanofi, Genentech/Roche, Eisai, AstraZeneca, Bristol-Myers Squibb, Amgen, Exelixis, EMD Serono, Merck, Seattle Genetics/Astellas; held stock/ownership interest in ECOM Medical; travel/accommodations/expenses paid by Genentech, Prometheus Laboratories, Astellas Medivation, Janssen, Eisai, Bayer, Pfizer, Novartis, Exelixis, and AstraZeneca; and received institutional research funding from Genentech, Exelixis, Janssen, AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Macrogenics, Astellas Pharma, beyond spring, BioClin Therapeutics, Clovis Oncology, Bavarian Nordic, Seattle Genetics, Immunomedics, and epizy.
YZ has served on advisory boards for Bristol Myers Squibb, Amgen, Roche Diagnostics, Novartis, Janssen, Eisai, Exelixis, Castle Bioscience, Array, Bayer, Pfizer, Clovis, and EMD serono; received institutional grant/research trial support from NewLink Genetics, Pfizer, Exelixis, and Eisai; served on the Data Safety Monitoring Committee for Janssen Research and Development, and received honorarium as a consultant for Pfizer and Novartis.
HP is a former employee of Calithera Biosciences.
LA and KO are employees and hold ownership interest in Calithera Biosciences.
NMT has consulted for Novartis, Exelixis, Bristol-Myers Squibb, Nektar, Pfizer, Eisai Medical Research, Ono Pharmaceutical, Oncorena, Surface Oncology, Neoleukin Therapeutics, Ipsen, Merck Sharp & Dohme, Calithera Biosciences; research funding from Bristol-Myers Squibb, Exelixis, Pfizer, Nektar Therapeutics, Calithera Biosciences, Lilly, Mirati Therapeutics, Arrowhead Pharmaceuticals, Takeda, Epizyme, Eisai; received honoraria from Pfizer, Novartis, Bristol-Myers Squibb, Exelixis, Nektar, Eisai Medical Research, Ono Pharmaceutical, Eli Lilly, Oncorena, Ipsen, Surface Oncology, Neoleukin Therapeutics, Merck Sharp & Dohme, Calithera Biosciences; and travel-related expenses from Pfizer, Nektar, Bristol-Myers Squibb, Eisai, Medical Research, Surface Oncology, Lilly Oncology, Ipsen, Calithera Biosciences.
IP, AH, VP, MS, BG, PN, and ZZ have no conflicts to declare.
Figures

References
-
- Razafinjatovo C, Bihr S, Mischo A, Vogl U, Schmidinger M, Moch H, et al. Characterization of VHL missense mutations in sporadic clear cell renal cell carcinoma: hotspots, affected binding domains, functional impact on pVHL and therapeutic relevance. BMC Cancer 2016;16:638 doi 10.1186/s12885-016-2688-0. - DOI - PMC - PubMed
-
- National Comprehensive Cancer Network. 2019 Kidney Cancer (Version 2.2020). <https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf>.
-
- Wells JC, Stukalin I, Norton C, Srinivas SL, J. L., Donskov F, Bjarnason GA, et al. Third-line Targeted Therapy in Metastatic Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2017;71(2):204–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

