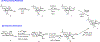Synthesis of a Diacetonide-Protected, Mannose-Based Oxepine: Configurational Control of Anomeric Acetate Activation
- PMID: 35576505
- PMCID: PMC10116867
- DOI: 10.1021/acs.joc.2c00206
Synthesis of a Diacetonide-Protected, Mannose-Based Oxepine: Configurational Control of Anomeric Acetate Activation
Abstract
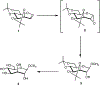
Carbohydrate-based oxepines are seven-membered-ring oxacycles containing an enol ether moiety. These compounds have been used as intermediates in the preparation of septanose carbohydrates by functionalization through their double bond. Reported here is a new synthesis of a carbohydrate based oxepine that uses 2,3;4,6-di-O-acetonide mannose as a key starting material. The oxepine is an important precursor used in the synthesis of septanose glycomimetics of mannopyranosides. The central feature of the synthesis is a two-step sequence that converts a septanose 1,2-di-O-acetate to the septanosyl bromide and onward to the oxepine via a reductive elimination.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Sinka V; Martín VS; Cruz DA; Padrón JI Synthesis of Seven Membered Oxacycles: Recent Developments and New Approaches. Eur. J. Org. Chem. 2020, 2020 (43), 6704–6717.
-
- Kleinke AS; Webb D; Jamison TF Recent Progress in the Synthesis of Oxepanes and Medium Ring Ethers. Tetrahedron 2012, 68 (35), 6999–7018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources