Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial)
- PMID: 35576508
- PMCID: PMC9426809
- DOI: 10.1200/JCO.21.02660
Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial)
Erratum in
-
Erratum.J Clin Oncol. 2022 Nov 10;40(32):3785. doi: 10.1200/JCO.22.02137. J Clin Oncol. 2022. PMID: 36343382 Free PMC article. No abstract available.
Abstract
Purpose: Neoadjuvant chemotherapy plus nivolumab has been shown to be effective in resectable non-small-cell lung cancer (NSCLC) in the NADIM trial (ClinicalTrials.gov identifier: NCT03081689). The 3-year overall survival (OS) and circulating tumor DNA (ctDNA) analysis have not been reported.
Methods: This was an open-label, multicenter, single-arm, phase II trial in which patients with stage IIIA NSCLC, who were deemed to be surgically resectable, were treated with neoadjuvant paclitaxel (200 mg/m2 once a day) and carboplatin (area under curve 6) plus nivolumab (360 mg) once on day 1 of each 21-day cycle, for three cycles, followed by adjuvant nivolumab monotherapy for 1 year (240 mg once every 2 weeks for 4 months, followed by 480 mg once every 4 weeks for 8 months). The 3-year OS and ctDNA analysis were secondary objectives of the trial.
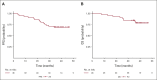
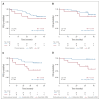
Results: OS at 36 months was 81.9% (95% CI, 66.8 to 90.6) in the intention-to-treat population, rising to 91.0% (95% CI, 74.2 to 97.0) in the per-protocol population. Neither tumor mutation burden nor programmed cell death ligand-1 staining was predictive of survival. Conversely, low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and OS (hazard ratio [HR], 0.20; 95% CI, 0.06 to 0.63, and HR, 0.07; 95% CI, 0.01 to 0.39, respectively). Clinical responses according to RECIST v1.1 criteria did not predict survival outcomes. However, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and OS (HR, 0.26; 95% CI, 0.07 to 0.93, and HR, 0.04; 95% CI, 0.00 to 0.55, respectively). The C-index to predict OS for ctDNA levels after neoadjuvant treatment (0.82) was superior to that of RECIST criteria (0.72).
Conclusion: The efficacy of neoadjuvant chemotherapy plus nivolumab in resectable NSCLC is supported by 3-year OS. ctDNA levels were significantly associated with OS and outperformed radiologic assessments in the prediction of survival.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 - PubMed
-
- Herbst RS, Baas P, Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 375:1823-1833, 2016 - PubMed

